- Adhesion to liquids
- Polymers
- Methods
- Tear-off method
- Delamination method
Adhesion is the bond between dissimilar surfaces brought into contact.
The reasons for the occurrence of an adhesive bond are the action of intermolecular forces or chemical interaction forces. Adhesion causes the bonding of solid bodies - substrates - with the help of an adhesive - adhesive, as well as the connection of a protective or decorative paint coating with the base. Adhesion also plays an important role in the dry friction process. In the case of the same nature of the contacting surfaces, we should talk about autohesion (authesion), which underlies many processes for processing polymer materials. With prolonged contact of identical surfaces and the establishment in the contact zone of a structure characteristic of any point in the volume of the body, the strength of the autohesive connection approaches the cohesive strength of the material (see cohesion). On the interfacial surface of two liquids or a liquid and a solid, adhesion can reach an extremely high value, since the contact between the surfaces in this case is complete. The adhesion of two solids due to uneven surfaces and contact only at individual points is usually small. However, high adhesion can also be achieved in this case if the surface layers of the contacting bodies are in a plastic or highly elastic state and are pressed against each other with sufficient force.
Adhesion, what it is - theoretical foundations
Adhesion is one of the key properties of materials in the following areas:
- Metallurgy – anti-corrosion coatings.
- Mechanics – a layer of lubricant on the surface of machine elements and mechanisms.
- Medicine - dentistry.
- Construction. In this industry, adhesion is one of the main indicators of the quality of work and the reliability of structures.
At almost all stages of construction, adhesion indicators for the following connections are monitored:
- paints and varnishes;
- plaster mixtures, screeds and fillers;
- adhesives, masonry mortars, sealants, etc.
An example of chemical adhesion - the reaction between silicone sealant and glass
There are three basic principles of adhesive bonding of materials. In construction and technology they manifest themselves as follows:
- Mechanical - adhesion occurs by adhesion of the applied material to the base. The mechanism of such a connection is the penetration of the applied substance into the pores of the outer layer or connection with a rough surface. An example is painting the surface of concrete or metal.
- Chemical - the connection between materials, including those of different densities, occurs at the atomic level. To form such a bond, the presence of a catalyst is necessary. An example of this type of adhesion is soldering or welding.
- Physical - electromagnetic intermolecular bonding occurs on mating surfaces. Can be formed as a result of a static charge or under the influence of a constant magnetic or electromagnetic field. An example of its use in technology is painting various surfaces in an electromagnetic field.
Adhesion Test Methods
Adhesion and autohesion when testing for peeling, shear and delamination can be determined using conventional dynamometers or special adhesiometers. To ensure complete contact between the adhesive and the substrate, the adhesive is used in the form of a melt, a solution in a volatile solvent or a monomer, which polymerizes when an adhesive compound is formed.
However, as the adhesive cures, dries, and polymerizes, it typically shrinks, resulting in tangential stresses at the interface that weaken the adhesive bond.
These stresses can be largely eliminated by introducing fillers, plasticizers into the adhesive, and in some cases by heat treatment of the adhesive joint.
The strength of the adhesive bond determined during testing can be significantly influenced by the size and design of the test sample (as a result of the so-called edge effect), the thickness of the adhesive layer, the history of the adhesive connection and other factors. Of course, we can talk about the values of adhesion or autohesion strength only in the case when destruction occurs along the interphase boundary (adhesion) or in the plane of the initial contact (autohesion). When the sample is destroyed by the adhesive, the resulting values characterize the cohesive strength of the polymer.
Some scientists believe, however, that only cohesive failure of an adhesive joint is possible. The observed adhesive nature of the destruction, in their opinion, is only apparent, since visual observation or even observation with an optical microscope does not allow one to detect the thinnest layer of adhesive remaining on the surface of the substrate. However, recently it has been shown both theoretically and experimentally that the destruction of an adhesive joint can be of a very diverse nature - adhesive, cohesive, mixed and micromosaic.
Adhesive properties of building and finishing materials
Adhesion of building and finishing materials is carried out mainly according to the principle of mechanical and chemical connection. A large number of different substances are used in construction, the performance characteristics and specific interaction of which are radically different. Let us divide them into three main groups and describe them in more detail.
paints and varnishes
Adhesion of paintwork materials to the surface of the base is carried out according to the mechanical principle. At the same time, maximum strength indicators are achieved if the working surface of the material is rough or porous. In the first case, the contact area increases significantly, in the second, the paint penetrates into the surface layer of the base. In addition, the adhesive properties of paintwork materials are increased thanks to various modifying additives:
- organosilanes and polyorganosiloxanes have an additional water-repellent and anti-corrosion effect;
- polyamide and polyester resins;
- organometallic catalysts for chemical processes of paint and varnish hardening;
- ballast fine fillers (for example, talc).
Talc-filled paint - non-intumescent fire retardant
Construction plasters and dry adhesive mixtures
Until recently, construction and finishing work was carried out using various mortars based on gypsum, cement and lime. Often, they were mixed in a certain proportion, which gave a limited change in their basic properties. Modern ready-made dry construction mixtures: starting, finishing and multi-finish plasters and putties have a much more complex composition. Additives of various origins are widely used:
- mineral - magnesia catalysts, liquid glass, aluminous, acid-resistant or non-shrink cement, microsilica, etc.
- polymeric - dispersible polymers (PVA, polyacrylates, vinyl acetates, etc.).
Such modifiers significantly change the following basic characteristics of building mixtures:
- plastic;
- water-retaining properties;
- thixotropy.
An example of poor adhesion of plaster to a brick wall
Important! The use of polymer modifiers gives a more pronounced effect of enhancing adhesion. However, the formation of stable compounds of polymer films at the interface of different types of materials (base - hardening plaster) is possible only at a certain temperature. This term is called the minimum film formation temperature - MTP. For different plasters it can vary from +5°C to +10°C. To avoid delamination, it is necessary to strictly adhere to the manufacturer's recommendations regarding temperature, both the environment and the substrate.
Sealants
Sealants used in construction are divided into three different types, each of which requires certain conditions for high-strength adhesion to the base material. Let's look at each type in more detail.
- Drying sealants. The composition includes various polymers and organic solvents: styrene butadiene or nitrile, chloroprene rubber, etc. As a rule, they have a paste-like consistency with a viscosity of 300-550 Pa. Depending on the viscosity, they are applied either with a spatula or a brush. After they are applied to the surface, a certain time is required for drying (evaporation of the solvent) and the formation of a polymer film.
Drying acrylic sealant
- Non-drying sealants. They usually consist of rubber, bitumen and various plasticizers. They have limited resistance to high temperatures, no more than 700C-800C, after which they begin to deform.
Non-drying bituminous composition, used for sealing storm drainage systems
- Curing sealants. After their application, under the influence of various factors: moisture, heat, chemical reagents, an irreversible polymerization reaction occurs.
Preparation of two-component polyurethane sealant Sazilast
Of all the listed varieties, curing sealants provide maximum reliability of adhesion to micro-irregularities of the base surface. In addition, they are resistant to high temperatures, mechanical and chemical influences. They have an optimal combination of rigidity and viscosity, allowing them to maintain their original shape. However, they are the most expensive and difficult to use.
For which materials is adhesion important?
This indicator is of primary importance for construction and finishing compositions. It is imperative to pay attention to the level of adhesion for the following types of coatings:
- Varnishes and paints. This property affects the quality of adhesion, penetration depth and durability of the coating. The higher the indicators, the better and longer the paint and varnish materials will last on the base.
- Gypsum mixtures. The quality of adhesion determines the possibilities of decorative finishing.
- Cement-sand compositions. The safety of the structure often depends on the reliability of gluing. For example, if you use substances with poor adhesion, the brickwork will not last long.
- Sealants and other adhesives. Here you need to know between which materials the product can ensure adhesion. When using unsuitable mixtures, the quality of the connection deteriorates, and in some cases becomes completely impossible.
USEFUL INFORMATION: Prime the ceiling before painting it with water-based paint
A special device, an adhesive meter, allows you to measure the adhesion ability of materials and control the quality of adhesion of the coating to the base.
How is adhesion measured?
The technology for measuring adhesion, testing methods, as well as all indicators of the bond strength of materials are specified in the following standards:
- GOST 31356-2013 - putties and plasters;
- GOST 31149-2014 - paints and varnishes;
- GOST 27325 - paints and varnishes for wood, etc.
Information! Adhesion is measured in kgf/cm2, MPa (megapascals) or kN (kilonewtons) - this is an indicator of the force that must be applied to separate the base and coating materials.
Method for determining the adhesion of paint and varnish coatings using the grid cut method
If previously the adhesion characteristics of materials could only be measured in laboratory conditions, now there are many instruments that can be used directly on the construction site. Most methods for measuring adhesion, both “field” and laboratory, are associated with the destruction of the outer, covering layer. But there are several devices whose operating principle is based on ultrasound.
Classification table for test results of paints and varnishes
- Knife adhesion meter. Used to determine adhesion parameters using the lattice and or parallel cuts method. Used for paint and film coatings up to 200 microns thick.
Knife adhesive meter, model Konstanta-KN2
- Pulsar 21. The device determines the density of materials. Used to detect cracks and delaminations in concrete, both piece and monolithic. There are special firmware and subprograms that, based on the tightness of the fit, allow you to determine the adhesion strength of various types of plasters to concrete surfaces.
Ultrasonic adhesion meter, Pulsar 21
- SM-1U. Used to determine the adhesion of polymer and bitumen insulating coatings by the method of partial destruction - shear. The measuring principle is based on identifying linear deformations of the insulating material. As a rule, it is used to determine the strength of the insulating coating of pipelines. It is allowed to use bitumen waterproofing on building structures to check the quality: walls of basements and ground floors, flat roofs, etc.
Adhesion meter SM-1U
What is adhesion in construction?
Adhesion is the adhesion of surfaces of dissimilar solids and/or liquids. I would also add – at the molecular level.
For example, tile adhesive with tiles and plaster or cement waterproofing. I just recently bonded ceresit cm17 glue with cement waterproofing and 1200*600 tiles:
It is important that before the adhesive was applied, the bases (plaster and tiles) were thoroughly coated with tile adhesive. This is just to improve adhesion.
Or adhesion of sealant to tiles:
I made this screenshot from Andrey Shaiter’s video. By the way, I highly recommend watching it - you will learn a lot of interesting things about sealants.
There is a fragment in the video where you can clearly see how the sealant breaks during a cohesive rupture:
Help from Wikipedia: Adhesion (from the Latin adhaesio - sticking)
In other words, when they mention adhesion, they mean the strength of adhesion at the points of contact of different materials, and when cohesion, it breaks in the middle (body) of the material.
I think the description clearly shows that when they talk about cohesion, we are not talking about adhesion.
Factors that reduce the adhesion of materials
Various physical and chemical factors influence the reduction of adhesion. Physical factors include the temperature and humidity of the environment at the time of applying decorative, finishing or protective materials. Various contaminants, in particular dust covering the surface of the base, also reduce adhesion interactions. During operation, ultraviolet radiation can affect the bond strength of paints and varnishes.
Chemical factors that reduce adhesion are represented by various materials that contaminate the surface: gasoline and oils, fats, acidic and alkaline solutions, etc.
Also, the adhesion of finishing materials can be reduced by various processes occurring in building structures:
- shrinkage;
- tensile and compressive stresses.
Information! A substance applied to the surface to increase the adhesion force between the base and the finishing material is called an adhesive. The base on which the adhesive is applied is called the substrate.
Causes of poor adhesion
If the paint or putty peels off, the reason is poor adhesion. Based on the nature of the damage, you can determine the cause of the defect:
- the surface is not prepared (poorly cleaned of dust, not degreased, poorly sanded);
- incompatibility of paint and varnish components with the previous coating of the base; paints do not adhere uniformly to aluminum and galvanized iron;
- the soil was diluted with a low-quality solvent;
- applying a thick layer of paint and varnish and other mixtures;
- the base enamel is overdried, in this case there is nothing for the varnish to cling to;
- incorrect proportions when preparing putties from dry mixtures;
- temperature conditions are not maintained.
Methods for increasing adhesion
In construction, there are several universal ways to increase the adhesion of decorative finishing materials to the base surface:
- Mechanical - the surface of the base is roughened to increase the contact area. To do this, it is treated with various abrasive materials, notches are applied, etc.
- Chemical – various substances are added to the applied protective and finishing materials. These are, as a rule, polymers that form stronger bonds and give the material additional elasticity.
- Physico-chemical - the base surface is treated with a primer that changes the basic chemical parameters of the material and affects certain physical properties. For example, reducing moisture absorption in porous materials, securing a loose outer layer, etc.
Treating the surface of the base before painting with abrasive sandpaper
Priming the surface before applying plaster
Liquid adhesion
Adhesion of liquid to liquid or liquid to solid. From the point of view of thermodynamics, the reason for adhesion is a decrease in free energy per unit surface of the adhesive joint in an isothermally reversible process. The work of reversible adhesive detachment Wa is determined from the equation: >Wa = σ1 + σ2 - σ12
where σ1 and σ2 are the surface tension at the boundary of phases 1 and 2, respectively, with the environment (air), and σ12 is the surface tension at the boundary of phases 1 and 2, between which adhesion takes place.
The adhesion value of two immiscible liquids can be found from the equation given above using the easily determined values of σ1, σ2 and σ12. On the contrary, the adhesion of a liquid to the surface of a solid body, due to the impossibility of directly determining σ1 of a solid body, can only be calculated indirectly using the formula:>Wa = σ2 (1 + cos ϴ)
where σ2 and ϴ are the measured values, respectively, of the surface tension of the liquid and the equilibrium contact angle formed by the liquid with the surface of a solid. Due to wetting hysteresis, which does not allow the contact angle to be determined accurately, only very approximate values are usually obtained from this equation. In addition, this equation cannot be used in the case of complete wetting, when cos ϴ = 1.
Both equations, applicable in the case when at least one phase is liquid, are completely inapplicable for assessing the strength of the adhesive bond between two solids, since in the latter case the destruction of the adhesive connection is accompanied by various types of irreversible phenomena due to various reasons: inelastic deformations of the adhesive and substrate, the formation of a double electrical layer in the area of the adhesive seam, the rupture of macromolecules, the “pulling out” of the diffused ends of the macromolecules of one polymer from the layer of another, etc.
Ways to increase adhesion to various materials
Let us dwell in more detail on methods for increasing adhesion for various materials used in construction.
Concrete
Concrete building materials and structures are widely used in construction. Due to the high density and smoothness of the surface, their potential adhesive properties are quite low. To increase the strength of the connection of finishing compounds, it is necessary to take into account the following parameters:
- dry or wet surface. As a rule, adhesion to a dry surface is higher. However, many adhesive mixtures have been developed that require pre-wetting the base surface. In this case, it is necessary to pay attention to the manufacturer’s requirements;
- ambient and substrate temperature. Most finishing materials are applied to concrete surfaces at an air temperature of at least +5°C...+7°C. In this case, the concrete should not be frozen;
- primer. Must be used. For dense concrete, these are compositions filled with quartz sand (concrete contact), for porous concrete (foam, aerated concrete), these are deep penetration primers based on acrylic dispersions;
- adding modifiers. Ready-made dry plaster mixtures already contain various adhesive additives. If the plaster is mixed independently, then it is recommended to add to it: PVA, acrylic primer, instead of the same amount of water, silicate glue, which gives the finishing material additional moisture-repellent properties.
The result of applying cement plaster to a supercooled base surface
Application of quartz primer Knauf betonkontakt
Metal
The method and quality of surface preparation plays a key role in the strength of the bond between paint and varnish materials and a metal surface. At home, it is recommended to do the following:
- degreasing - treating metal with various solvents: 650, 646, R-4, white spirit, acetone, kerosene. As a last resort, the surface is wiped with gasoline;
- matting – processing the base with abrasive materials;
- priming - using special primer paints. They are sold complete with decorative paintwork materials of a certain type.
Important! The adhesion of lead, aluminum and zinc is much lower than that of cast iron and steel. The reason is that these metals form oxide films on their surface. Therefore, peeling of paint coatings occurs along the oxide layer. It is recommended to paint these materials immediately after removing the film mechanically or chemically.
Aluminum is also susceptible to corrosion, especially when exposed to aggressive substances
Wood and wood composites
Wood is a porous surface with a large number of irregularities and does not experience any special problems with the strength of the connection of finishing materials. But there is no limit to perfection, so various technologies have been developed to improve adhesion while maintaining the protective and decorative properties of the finish itself. Their use, for example, in combination with acrylic paints, significantly improves weather resistance, resistance to ultraviolet fading, and imparts biological protection to the material. The wood surface is treated with a wide variety of primers, most often based on boron-nitrogen compounds and nitrocellulose.
Analysis of forces acting during adhesion
The question of the nature of adhesion phenomena is currently of particular importance due to the widespread use in industry of gluing various materials using high-molecular compounds. To understand the chemical and physical phenomena underlying the bonding processes, let us familiarize ourselves with existing theories and terminology.
Adhesion, or adhesion , is the connection between the surfaces of two dissimilar solid or liquid bodies, caused by the forces of intermolecular interaction, i.e., the forces of adhesion of dissimilar molecules, atoms, ions, functional groups located in the surface layers of contacting bodies. These adhesive forces are called adhesive forces, and the interaction itself is called an adhesive interaction.
In the case of contact between two solid bodies, adhesion is very small due to the fact that the actual area of their contact due to surface irregularities is very small in relation to the entire contact area.
When two liquid bodies or a solid and a liquid come into contact, adhesion at the interface can be significant under certain conditions. The same result is achieved as a result of contact of solids in a plastic or elastic state at high pressure.
Adhesion is determined by the work, or resistance to rupture, under a corresponding deformation (pull or shear). The work spent on overcoming the adhesion forces when separating particles of two dissimilar surfaces is called the work of adhesion, or the work of separation.
Adhesion, or adhesion, is the connection between the surfaces of two homogeneous bodies, and the adhesive forces arising in this case are called adhesion forces. An example of adhesive interaction is the adhesion of films of a sizing agent to each other.
Cohesion is the adhesion of molecules, atoms, and ions of a substance to each other in the volume of a body - an adhesive substance, materials being glued together. If the contacting surfaces are identical, homogeneous, adhesion turns into cohesion, which characterizes the strength of these bodies, the force of adhesion of their molecules, atoms, and ions. The work expended against the cohesive forces that act between particles within a given body is called cohesive work. Solids have much greater cohesive forces than liquids, but gases have none.
Cohesion is determined by various forces—van der Waals forces, chemical bonds (covalent and electrovalent, hydrogen bonds, etc.). These forces are called cohesive forces.
To ensure bonding strength, it is necessary to use adhesive materials with high adhesive and cohesive properties.
The bonding strength of dissimilar surfaces is determined by the ratio of adhesion and cohesion forces: adhesion must be greater than cohesion. If, with sufficiently high cohesion, the adhesion forces are greater than the cohesive forces, the bonding is strong and, conversely, if the cohesive forces prevail over the adhesion forces, the bonding is weak.
If the cohesive forces of the adhesive are greater than the forces of its adhesion, for example, to wood, then there is a danger of separation of the adhesive layer from the wood being glued.
If an external force is applied to an adhesive joint with sufficiently high adhesion, the rupture should occur not along the adhesive seam, but cohesively, i.e., inside the weakest of the conjugate bodies.
Both adhesive and cohesive properties of a substance are determined by the nature of interatomic and intermolecular bonds, the size and spatial structure of their particles. Cohesion largely depends on the size and shape of the molecules and other chemical properties of the adhesive. The cohesion of the adhesive layer characterizes its mechanical properties - strength, rigidity and wettability.
Wettability refers to the ability of liquid glue to spread over the surface of a solid as a result of the action of attractive forces between particles of liquid and solid matter. In other words, when a solid surface is wetted with a liquid at the phase contact boundary, the phenomenon of adsorption , the essence of which is that a layer of liquid molecules is adjacent to the solid surface. Therefore, all adhesives, regardless of their initial physical state, must have a certain ability to wet the materials being glued during the gluing process. This is necessary to ensure maximum contact between the adhesive and the material being glued and their subsequent interaction during the gluing process.
Good wettability of the glued material by the adhesive entails the formation of a strong, and poor wettability leads to the formation of a weak adhesive joint. With good wetting, the tensile force is distributed evenly in the adhesive joint; with poor wetting, the stresses arising during the hardening process are concentrated at individual points, where, with insignificant external forces, the adhesive joint is destroyed.
An example illustrating the impossibility of gluing materials that are not wetted by liquid glue can be synthetic adhesives with active, functional groups in chain molecules (-OH, -COOH, -CN). These adhesives practically do not wet the surfaces of materials with low surface energy (such as polyethylene, polypropylene) and therefore do not glue them at all. At the same time, materials with high surface energy (metals, quartz, silicate glass), which are well wetted by the same adhesives, provide high-strength connections. However, wetting itself is not the only condition that determines the degree of adhesion of the adhesive to the material and the strength of the adhesive joints.
To bond inert polymers with low surface energy to metals and other materials, their surface is first chemically modified by chemical reactions. As a result of chemical action, adhesion-active functional groups (–OH, –COOH, –NH2) are formed in the macromolecules of the surface layer, promoting the adhesion of polymers to metals and other materials.
Polar polymers containing a large number of chemically active functional groups (hydroxyl, carboxyl, etc.) exhibit fairly high adhesion to materials whose molecules also contain polar groups. Non-polar (hydrocarbon) polymers, such as polyethylene, polypropylene, polyisobutylene, exhibit low adhesion to polar polymers.
To clarify the adhesive ability of polymers depending on their polarity, let us consider some properties of the molecules of substances.
It is known that a molecule consists of a relatively heavy, positively charged nucleus and much lighter negatively charged electrons. The molecule is thus an electrical system consisting of positive and negative charges. In general, the molecule is electrically neutral, since the negative charges of its electrons are compensated (balanced) by the positive charges of the nuclei.
Molecules of various compounds are divided into polar, in which electric charges of the opposite sign are not completely compensated and their centers of gravity are applied in different places inside the molecule, and non-polar.
Polar substances include water, acids, alcohol, cellulose, liquid phenol and urea-formaldehyde resins, etc.
Polar molecules having electric charges equal in magnitude but opposite in sign, located at a certain distance from each other, are called dipoles. The product of the magnitude of these charges and the distance between them is called the dipole moment, which characterizes the polarity of the molecules.
Polar molecules are characterized by a relatively high dielectric constant. The attractive forces acting between polar molecules are largely due to the so-called orientational moment (the molecules are oriented towards each other in a certain way due to the action of their electrical charges). In this case, a dependence of the adhesion forces and dielectric constant on temperature is observed.
The cohesion between polar molecules is called orientational interaction .
Mutually attracted by their oppositely charged poles, polar molecules always strive to take a position in relation to one another or to the polar molecules of other substances in which their positive and negative charges are neutralized. Under these conditions, the potential energy on the contact surface turns out to be minimal, and the greater its decrease, the greater, other things being equal, the adhesion forces turn out to be greater.
Non-polar molecules include molecules of many substances that have a symmetrical structure, for example hydrogen H2, nitrogen N2, oxygen O2, carbon dioxide CO2 and many others. In non-polar molecules in their natural state, the electrical centers of gravity of the positive charges of the nucleus and the negative charges of the electrons coincide. Non-polar substances have low dielectric constant.
When polar and nonpolar substances come into contact, nonpolar molecules can become polarized under the influence of polar molecules in close contact with them, resulting in induced dipoles.
The interaction of induced dipoles with dipoles of polar molecules also leads to mutual attraction, but weaker than in the case of interaction of dipoles of polar substances. This interaction of polar and non-polar molecules is called inductive interaction.
There are also intermolecular interaction forces that are exhibited by molecules of all substances - polar and non-polar. These forces are also electrostatic in nature. These include van der Waals forces and dispersion forces.
Van der Waals forces are the forces of mutual attraction of molecules (or repulsion of them when very close to one another).
Dispersion forces are forces of mutual attraction between molecules or atoms that act over large distances.
Unlike orientation forces, inductive and dispersion forces do not depend on temperature.
Adhesion, however, is not only determined by the above factors. Depending on the nature of the materials being glued and the adhesives, various types of chemical bonds may manifest themselves during adhesion, for example, heteropolar or ionic, homeopolar or covalent, semipolar or coordination bonds, as well as hydrogen bonds.
An ionic bond occurs between oppositely charged ions. The formation of an ionic bond between two atoms occurs as a result of the transfer of electrons from one atom to another. Both atoms thus turn into ions with opposite electrical charges. Due to mutual attraction, the ions combine with each other to form neutral molecules. The simplest example of an ionic bond is the bond between a positive sodium ion and a negative chlorine ion in a molecule of table salt.
A covalent bond is formed between two neutral atoms by each donating one or more electrons to form electron pairs shared by both atoms.
A semipolar bond is characterized by some displacement of common electron pairs towards one atom. By its nature, this bond occupies an intermediate position between ionic and covalent.
Most synthetic adhesive resins are characterized by a covalent bond.
Hydrogen bonding occupies a special position in bonding processes. It represents a specific interaction between molecules containing hydrogen in the O - H groups; N - N; Cl - H; F - N.
Under the influence of hydrogen bonds, the association of molecules of substances such as alcohol, water, and acid occurs. Hydrogen bonds are involved in the formation of spatial structures during the curing process of some polymers (phenol-formaldehyde resins). The properties of many polar polymers (cellulose, etc.) are also determined by hydrogen bonds.
Adhesion during welding
Welding is one of the most durable methods of joining metal structures. This is the adhesion of molecules of two elements without the use of intermediate or auxiliary substances - glue or solder. This process occurs under the influence of thermal activation. The outer layer of the elements being joined is heated above the melting point, after which intermolecular rapprochement and joining of materials occurs.
Electric welding seam. The connection of two parts by electric welding is adhesion, since the metal used in the electrode acts as an adhesive
The following factors can serve as an obstacle to high-quality adhesion during welding:
- presence of oxide films. They are removed mechanically or chemically during surface preparation or disappear directly during the welding process under the influence of high temperatures or fluxes;
- mismatch of the chemical composition of materials and electrodes. Particular attention should be paid to the presence and amount of silicon and carbon in the parts being joined. To connect steels of different grades, it is recommended to use electrodes with a low diffusible hydrogen content;
- insufficient penetration depth, which directly depends on the current strength and the speed of movement of the electrode.
Gas or plasma welding of metal is cohesive, since the molecules of two elements are combined as a result of the melting of the material
Consequences of poor adhesion
The consequences of weak adhesion can be clearly seen during painting work. The process can manifest itself differently on different layers. Detachment of soil from the base occurs if the soil is too dry or applied in a thick layer. The reason for paint peeling from the primer is due to incompatibility of materials or delay in paint application. When adhesion is weak, layers of paint begin to separate; this process can affect one or all layers. The consequences may appear immediately, after 2-4 days, or even after a couple of months.
Composition of the adhesive system
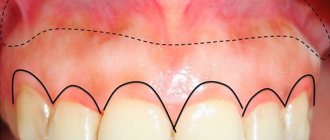
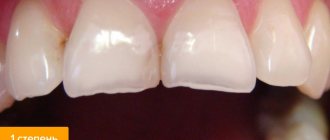
The system used in dentistry today has several components.
- Etching composition or etchant. It is an organic or inorganic acid. It is needed in order to remove all organic matter from the surface being treated, and also creates special micropores for better adhesion. Acids that can be used are orthophosphoric, citric, maleic, etc.
- Bond is the adhesive itself, which has a rather complex chemical composition. It contains methacrylates, solvents, as well as special stabilizers and a number of other substances. Designed to ensure bond between the composite and the tooth surface.
- Primer is a substance that contains hydrophilic components, as well as various stabilizers, etc. It is needed to prepare dentin for processing and allows you to create a so-called hybrid layer. Without this substance, the adhesion of dental materials to dentin is almost impossible.
- Filler is an inorganic substance that is present in the primer and in the adhesive itself. Makes it possible to achieve a durable hybrid layer when processing a tooth.
- Solvents can be either water, alcohol or acetone. They make it possible to leave working materials liquid and flowing.
- An activator is an additional component that is necessary when working with dual-curing substances, as well as for working in the field of orthopedics. It is added to the bond or primer to cure the adhesive system.