Author: M. Cannon, N. Gerodias, A. Vieria, S. Persinoto, R. Yurado, Northwestern University School of Medicine, Department of Surgery, Children's Memorial Medical Center, Chicago, USA, San Paolo State University, Department of Pediatric Dentistry and endodontics, Aracataba, Brazil
Minimally invasive dentistry requires the use of conservative methods of restoring the dentition. But it is necessary to remember about the complications that may arise after this manipulation. The purpose of this nonhuman primate study was to compare the effectiveness of a novel light-curing pulp cap, TheraCal, Portland cement, synthetic calcium hydroxide, and glass ionomer in the treatment of bacterially infected pulp in primates.
Summary
The concept of minimally invasive dentistry is to use primarily conservative methods of dental restoration. This approach involves removing only those tooth tissues that cannot be restored, and relies more on the use of adhesive technologies rather than creating retention of the filling through a specific cavity design. However, even the use of the most modern adhesives does not exclude the possibility of marginal microleakage, which jeopardizes the vitality of the dental pulp.
In addition, even with gentle preparation, there is a risk of damage to the pulp. This may be facilitated by the anatomical and histological features of the teeth, such as a large pulp chamber, pronounced pulp horns or insufficiently formed, defective dentin. In such cases, maintaining the viability of the damaged pulp may require the use of a direct capping material that promotes pulp healing and the deposition of reparative dentin. Before suggesting the use of any of these materials in humans, the effectiveness of their use in animal studies should be assessed.
The purpose of this study was to compare the effectiveness of a new light-curing direct pulp capping material, pure Portland cement, polymer calcium hydroxide, and glass ionomer cement in the treatment of bacterially contaminated pulps in primates. Four individuals were included in the experiment, each of which had 12 teeth prepared under general anesthesia, exposing approximately 1.00 mm of pulp tissue on the buccal side. Cotton swabs soaked in a bacterial mixture consisting of microorganisms typically found in infected pulp were then applied to the exposed areas of the primate dental pulp. After removal of the packing, hemorrhage control and direct pulp capping were performed. The light-curing polymer material was applied using a needle and syringe to the exposed pulp areas of twelve teeth and then light-cured for 15 seconds. Pure Portland cement mixed with a 2% chlorhexidine solution was applied to the exposed pulp tissue of the other twelve teeth. An additional twelve teeth were treated with Triage Fuji VII glass ionomer cement (GC America), and the pulp of the remaining twelve teeth was capped with VLC DYCAL (Dentsply), a light-curing calcium hydroxide resin material. Fuji II resin-modified glass ionomer cement (GC America) was then applied to the base layer covering the exposed pulp. After 4 weeks, after the animals were killed, samples of the treated teeth were collected. These samples were demineralized, divided into fragments, stained and distributed into groups according to histological data. Statistical analysis of the obtained results was performed using the Kruskal-Wallis test.
When assessing inflammatory changes in the pulp, no statistically significant differences were found between groups (H = 0.679 with three degrees of freedom, P = 1.00). However, in both the Portland cement and light-curing pulp capping groups, hard tissue barrier formation was significantly more common by day 28 than in the GIC and Dycal groups (H=11.989 with three degrees of freedom, P=0.009). In addition, the thickness of the dentinal bridge in the first two groups was significantly greater than in the other two (H = 15.849 with three degrees of freedom, P = 0.002). The incidence of pulp necrosis in the GIC group was higher than in the other groups. Encouraging results achieved with a new light-curing material for direct pulp capping led to the development of TheraCal. Dentists quickly introduced TheraCal into their clinical practice as an easy-to-apply light-curing material for direct pulp capping. TheraCal does not interfere with standard techniques primarily used in clinical practice. Therefore, TheraCal can be successfully used as part of a dental restoration scheme using adhesive technologies, widely used in minimally invasive dentistry.
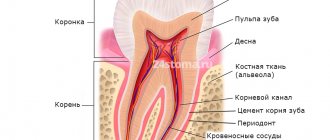
Conservative (biological) method of treating pulpitis (without removing the pulp)
The biological method is based on the properties of the pulp, after research of which its ability to recover was proven. Based on the plastic capabilities of the pulp, the conservative method of treating pulpitis aims to completely eliminate inflammation in the pulp and restore its biological function. The method is used in the initial phase of development of the inflammatory process or in case of accidental opening of the pulp; treatment should be started quickly to prevent inflammation from progressing to a more severe stage.
Indications and contraindications of the biological method
Conservative technique is indicated in the following cases:
- Pulp hyperemia is a condition caused by the expansion of the lumens of the blood vessels of the pulp. Accompanied by minor inflammation and increased sensitivity to cold irritants. Pulp hyperemia occurs when deep carious cavities appear, during tooth treatment for a crown, or when the technology for installing composite fillings is not followed. Hyperemia is a reversible process, therefore only a biological method is used to treat it.
- Accidental exposure of the pulp can occur during the preparation of a deep carious cavity, as well as as a result of tooth trauma. The conservative method in this case is used if there is no prolonged bleeding from the tooth cavity; if no more than a day has passed since the tooth was injured and the pulp was exposed.
- Acute pulpitis of a limited nature is the initial stage of the process, in which the integrity of the walls of the blood vessels of the pulp is preserved.
Biological pulp treatment is performed in the presence of the following additional conditions:
- The patient's age is not older than 30 years (there are exceptions). This method is often used to treat permanent teeth that have not yet formed roots.
- No changes in the periodontium confirmed by x-ray examination.
- There are no signs of periodontitis development.
Contraindications to biological pulp treatment:
- Significant reduction in electrical excitability of the pulp (indicators of electrical excitability differ for single-rooted and multi-rooted teeth, for different age categories of patients).
- Changes in the periodontium.
- Signs of changes in periapical tissues.
- Using a tooth as a support for a bridge.
- A carious cavity is located in the area of the neck or root of the tooth.
Methodology for biological pulp treatment
1. Indirect pulp capping method - used when there is a thin layer of healthy or partially decalcified dentin, which protects the pulp from exposure and injury. Treatment is carried out in the following sequence:
- Local anesthesia.
- Removal of tissues affected by caries.
- Apply a calcium hydroxide-based medicinal dressing to the remaining layer of dentin. The composition of therapeutic dressings may include antibacterial agents, enzymes, vitamins and glucocorticoids in order to accelerate the elimination of inflammation and stimulate regeneration.
- Placement of a temporary filling.
- A week later - a second visit. If the patient has no complaints, the temporary filling is replaced with a permanent one. If the pain persists, apply a new therapeutic bandage for several days; if the pain does not disappear after that, surgical treatment of pulpitis is indicated. In some cases, the period between the installation of a temporary and permanent filling can be up to 6 months.
2. Direct pulp capping method - used on accidentally exposed pulp. The medicinal dressing is applied directly to the exposed pulp; under the influence of medications, its plastic function is restored - dentin begins to form. An important point when applying a therapeutic dressing is careful removal of the blood clot formed during bleeding during exposure of the pulp. If this is not done, the clot will prevent contact of the dressing with the pulp, making treatment more difficult.
Introduction
An ideal material for direct pulp coating should have a bactericidal effect, should not have a negative effect on the pulp and other tissues of the oral cavity, and should activate regenerative processes in the pulp. Various substances have been proposed for these purposes, such as lyophilized bone, collagen, morphogenetic proteins and allogeneic dentin matrix, as well as non-pharmacological treatment methods, such as laser and electrosurgery. The properties of calcium hydroxide, such as biocompatibility, the ability to stimulate the formation of a mineralized barrier, a high acidity level and the associated bactericidal effect, also allow it to be considered as a material for direct coating of dental pulp.
Loma Linda University, California, has proposed a new drug called mineral trioxide aggregate (MTA) designed to close communications between the root canal system and the outer root surface at various levels. Mineral trioxide aggregate consists of small hydrophilic particles (powder) that harden in the presence of moisture. Its main components are: tricalcium aluminate, tricalcium silicate, calcium oxide and silicon oxide. All of these substances are constituents of Portland cement, with the exception of bismuth oxide, which was added to MTA to impart radiopaque properties. Using electron microanalysis, it was established that the main ions present in MTA are calcium cation (Ca2+) and phosphate anion (PO43–), and since they are also the main components of dental tissue, the latter are biologically compatible with this drug. Once mixed with water, the material's acidity level increases from pH 10.2 to pH 12.5 over the next three hours, giving it antimicrobial properties against certain facultative anaerobic bacteria. This drug is characterized by excellent sealing properties, biocompatibility, lack of mutagenic activity, low cytotoxicity, and the ability to stimulate a cellular response, resulting in the deposition of cement on the root surface, which promotes the regeneration of the periodontal ligament and the formation of bone tissue.
Holland and colleagues showed that MTA, which contains, among other things, calcium oxide, acts similarly to calcium hydroxide: when the powder is mixed with water, calcium oxide is converted to calcium hydroxide. Upon contact with tissue fluids, calcium hydroxide dissociates into Ca2+ and OH– ions. Calcium ions react with carbon dioxide in tissues, causing the deposition of limestone granules. In parallel with the deposition of the mineral component, fibronectin accumulates, which promotes cell adhesion and differentiation. Over time, a hard tissue barrier forms.
Pitt Ford and co-workers studied the response of pulp tissue after coating it with MTA and Dycal and found the presence of dentinal bridges after 6 months in the pulp of all teeth treated with MTA, while in the group where Dycal was used, all teeth showed signs of severe chronic inflammation and only two cases of dentin bridge formation. Abedi et al found that MTA resulted in less inflammatory response and greater calcification than Dycal for direct pulp capping in dogs and monkeys. Previous studies have demonstrated the formation of dentin bridges following direct pulp capping with VLC Dycal, a light-curing, polymer-based material containing calcium hydroxide. Pitt Ford and Roberts studied the response of the pulps of 64 teeth of four cynomolgus monkeys to mechanical exposure followed by direct coating (immediately or after 24 hours) of Dycal, VLC Dycal, or Prisma-Bond. Dentin bridge formation was found in almost all teeth treated with Dycal or VLC Dycal, and pulp inflammation was observed in only one tooth, indicating infection. However, the success rate in the delayed pulp capping group was as high as in the immediate capping group.
Holland showed that pulp capping with both MTA and Portland cement promoted the formation of dentinal bridges after pulpotomy in dogs. Estrela reports that MTA and Portland cement have comparable antibacterial activity. Saidon and Menezes also showed that the use of MTA and Portland cement as direct pulp capping materials produced similar histological results. It was therefore no surprise that Funteas found no significant differences when comparing 14 different elements of Portland cement and MTA. De Deus and co-workers compared the cytotoxicity of ProRoot MTA and MTA Angelus with Portland cement and found no statistically significant differences between the materials.
Upcoming events
2021-09-01BASIC COURSE ON DENTAL IMPLANTATION WITH DR. FRIEDMAN FOR STUDENTS AND RESIDENTS (Annual course)
2022-01-26Predictable results of orthopedic treatment in digital format.
2022-02-05International Implantology Congress
Indicators of reversible inflammation:
- Uniform width of the periodontal ligament space.
- No history of pain for less than 1 minute.
- Lack of sensitivity when clenching teeth and eating.
- No pain during percussion and a positive reaction to thermal stimuli.
Indicators of irreversible pulpitis:
- Spontaneous pain or provocative pain that lasts minutes to hours after the stimulus is removed.
- Pain on percussion.
- The expansion of the space of the periodontal ligament is an important criterion that cannot always be objectively assessed.
- Lack of response to stimuli.
- The presence of pus with symptoms of reversible pulpitis.
An important point is the patient’s participation in making the choice of treatment method, his understanding of the risks and complications. Nowadays it is very important for dentists to develop their Soft skills - communication skills with patients. It is important to tell the patient about the benefits of the treatment method.
Advantages of direct pulp capping:
- efficiency;
- rapidity;
- the ability to preserve more healthy tissue and a natural defense mechanism against the penetration of microbes into the tooth, therefore increasing the life of the tooth;
- simplicity compared to endodontic treatment.
But since everything is so good, why do we know little or do not use this technique in adult patients? Firstly, the relative complexity of the manipulation and the need to know the technique. Secondly, the difficulty of assessing the criteria for the reversibility of pulpitis, since in any case contamination of the pulp is possible. In addition, the necessary materials and equipment must be available. And finally, dynamic monitoring of such patients is necessary.
We should also not forget about the possible formation of replacement dentin in the tooth cavity, the development of dystrophic calcification in the root canal and the increased complexity of endodontic treatment (if required in the future). All this leads to controversy and ambiguity in the choice of this technique, especially in adult patients.
Treatment of pulp exposed to the oral cavity due to caries or trauma has been a dental specialty for several centuries. For a long time, attempts to preserve exposed pulp using conservative methods, such as capping or pulpotomy, were considered hopeless. However, over the past 70 to 80 years it has become clear that pulp healing after opening has become possible. This change in concept occurred due to the observation of regeneration of the exposed area when treating a pulp wound with water-based calcium hydroxide [5].
Materials and methods (study on primates)
The experiment included four individuals, young male Capucin Cebus Opella, which were randomly selected from the primate population of the Monkey Research Center, Aracataba, Sao Paolo. The study design was approved by the Animal Research Committee of the University of São Paulo, Aracatuba, Brazil. Throughout the experiment, animals were cared for in accordance with international standards. All procedures were performed in the primate operating room of the Monkey Research Center. According to the established research plan, 12 teeth were prepared from each primate with penetration into the pulp tissue from the buccal side. The dissection was carried out under general anesthesia with thiopental 30 mg/kg intramuscularly and diazepam 17 cm3 intramuscularly under sterile conditions. The teeth were previously cleaned of plaque and isolated using a rubber dam. Disinfection of the surgical field was carried out with providone iodide. Tooth preparation was carried out using small high-speed handpieces (Dabi Allante). Before each use, the tips were autoclaved. A new sterile small spherical bur was used for preparation. The surgical field was cooled with a sterile isotonic solution. The cavity was formed within the enamel, expanding it in the mesiodistal direction. The preparation was completed by opening the pulp chamber in the center of the formed cavity in an area with a diameter of 1.0 mm (Fig. 1). After completion of the preparation, the exposed pulp tissue was irrigated with a sterile isotonic solution to remove dentinal filings.
| Rice. 1. Isolation, disinfection and preparation of primate teeth. An area of exposed pulp measuring approximately 1.0 mm is located in the center of the prepared cavity |
Cotton swabs soaked in a bacterial mixture consisting of anaerobic and aerobic microorganisms typically present in infected pulp were placed on exposed areas of primate dental pulp. This mixture contained Porphyromonas gingivalis and Fusobacterium nucleatum, since both of these species are known to cause acute pulpitis and apical abscesses. After bacterial inoculation (with an exposure duration of 30 min), the pulp tissues were immediately washed with a sterile isotonic solution. Cotton swabs soaked in Cipro HC Otic solution were then applied to the infected pulp for 5 minutes. After removal of the swabs containing the solution, bleeding control and direct pulp capping were performed.
Light-curing polymer cement for direct pulp capping was applied to the pulp of 12 teeth at the tip of a syringe needle and light-cured for 15 s. Pure Portland cement mixed with a 2% chlorhexidine solution was applied to the exposed pulp of the other 12 teeth. An additional 12 teeth were treated with GIC (Triage, Fuji VII, GC America), and the pulp of the remaining 12 teeth was coated with VLC DYCAL (Dentsply), a light-curing resin material containing calcium hydroxide. Polymer-modified GIC (Fuji II LC, GC America) was then applied to the base layer of direct pulp capping material.
All monkeys were cared for during the experiment in accordance with international animal care standards. The primates were monitored for any changes in eating habits or signs of inflammation or suppuration of the oral tissues. At the discretion of the animal care staff, analgesics were administered when necessary. The behavior of the primates was carefully observed, and all data was recorded. No changes in behavior were observed in any of the animals.
4 weeks after the animals were killed, samples of the prepared teeth were collected and serial sections of tissue 6 mm thick were carried out using a Leica BM 2025 microtome. The preparations were stained using the following methods: hematoxylin and eosin staining, brown and brown staining, Masson trichrome staining . All specimens were submitted to Northwestern University for independent histological evaluation. Histological examination of the specimens was carried out using a Leitz Dialux 20 microscope. The personnel conducting the independent assessment were not aware of what materials and techniques were used, since all specimens were identified only by location. Histological analysis was carried out according to the following parameters: • the presence of necrosis, hyperemia, • the thickness and quality of the hard tissue barrier, • the presence of odontoblasts, the presence of other foci of calcification, • the presence of gigantocytes, • the presence of particles of pulp covering material (Table 1). The severity of inflammatory changes was assessed on the following scale: • no signs of inflammation – 0, • mild inflammation – 1, • moderate inflammation – 2, • severe inflammation – 3, • abscess formation – 4.
Table 1. Results of histological examination
| Inflammation scale | TheraCal | Portland cement | GIC | Dycal |
| 0 | 7 | 4 | 3 | 2 |
| 1 | 1 | 4 | 1 | 2 |
| 2 | 1 | 2 | 3 | 4 |
| 3 | 1 | 1 | 1 | 3 |
| 4 | 1 | 1 | 3 | 0 |
Dentin bridge thickness was measured (Fig. 2) using a phase contrast microscope using three randomly selected points on two different sections of each specimen. Statistical processing of the data was carried out by independent specialists.
| Rice. 2. Example of a histological specimen after application of a light-curing pulp capping material showing measurement of the dentinal bridge. Odontoblast-like cells accompanying the bridge are also visible, and there are no signs of inflammation |
Surgical methods for treating pulpitis
Vital amputation
Another name for the procedure is vital pulpotomy . The technique consists of performing a partial depulpation operation, that is, removing an area of the pulp susceptible to inflammation. The coronal pulp is removed, while the viability of the root pulp is preserved. This method is important in the treatment of pulpitis in multi-rooted teeth with immature roots.
Sequence of pulpotomy:
- Pain relief with injection of local anesthetic.
- Removal of tissues affected by caries.
- The tooth cavity is then opened and exposed to allow access for the next step.
- The dentist performs a pulpotomy - removes the coronal part of the pulp.
- If bleeding from the pulp occurs, it is stopped using tampons or a hemostatic sponge.
- A medicated pad with calcium hydroxide is applied to the root part of the pulp.
- Applying an insulating gasket and installing a temporary filling.
- After 1-4 weeks, replace the temporary filling with a permanent one (after confirming the viability of the pulp using electroodontodiagnostics).
Vital extirpation
Another name for the procedure is vital pulpectomy . As a result of using the technique, the pulp is completely removed. The method is used for all forms of pulpitis, as well as after a pulpotomy, when the inflammatory process continues to develop in the root part of the pulp. Depulpation using the vital extirpation method is carried out in one visit to the dental office.
Sequence of the procedure:
- Local anesthesia. Pulp anesthesia is provided by infiltration, conduction, intraligamentary anesthesia, their combination, or, less commonly, general anesthesia. Sometimes intrapulpal anesthesia is used. The operation to completely remove the pulp lasts 1-1.5 hours, so anesthetics must be guaranteed to provide anesthesia for this period.
- Preparation. Preparation of a carious cavity (removal of pathologically altered tooth tissues - enamel and dentin) is carried out to create access to the root canals. In other words, the dentist performs caries treatment
- Opening of the tooth cavity. When properly opened, the tooth cavity should merge with the carious cavity, without forming overhangs or bends at the border. The opening and development of the cavity is carried out in such a way that its walls smoothly transition into the walls of the tooth cavity;
- Pulpotomy. Amputation of the coronal part of the pulp, access to the mouths of the root canals is prepared;
- Expansion of canal mouths. At this stage, conditions are created for further endodontic treatment.
- Pulpectomy. The root pulp is removed; for this purpose, a pulp extractor is used - an endodontic instrument that is inserted into the root canal; when it is rotated around its axis, it engages with the pulp and is removed along with it. It is important that there are no pulp particles left in the root canal, as this causes infection and the development of residual pulpitis and periodontitis.
- Channel research. It is carried out by probing them with root needles, drills and drills with limiters. The exact parameters of the canal during the study (working length of the canal) are obtained using an apex locator and a visiograph;
- Stop bleeding. If bleeding occurs during pulpectomy, a turunda impregnated with a hemostatic agent is inserted into the root canal. Bleeding is also stopped using diathermocoagulation - a needle is inserted into the root canal, which is an active electrode, and an electric current is applied with high frequency, high strength and low voltage. As a result of this effect, blood clotting occurs, bleeding stops and necrosis of the remaining pulp occurs.
- Mechanical and medicinal treatment of canals. At this stage, the dentist must completely remove remaining pulp and infected tissue from the canals and prepare them for filling by giving them a cone shape. In addition to the mechanical treatment of each canal with special tools, medicinal treatment is carried out: the canal is washed with antiseptic solutions or cotton wool soaked with medicinal substances is inserted into it. Solutions used: sodium hypochlorite, furatsilin, chlorhexidine, hydrogen peroxide.
- Drying of the root canals is carried out with turundas soaked in alcohol and then ether and air;
- Filling canals, for example, using three-dimensional obturation using liquid gutta-percha. It is performed using special filling materials, which have many requirements: they must not stain the tooth over time, fit tightly to the canal walls, be radio-opaque, do not shrink, not cause irritation to surrounding tissues, and much more. Filling the root canal is a very important final stage; the success of endodontic treatment depends on the quality of filling the canal. Filling of canals is carried out with gutta-percha - this material best meets the requirements for modern materials for filling root canals, and therefore provides a reliable treatment result. The quality of canal filling is checked using an x-ray image - the canal must be completely sealed, without voids.
- Installation of a temporary filling.
- Installation of a permanent filling. It is carried out a few days after the installation of a temporary filling. Excess canal-filling material is removed from the canal, an insulating gasket and a permanent filling are applied.
Treatment of pulpitis in Dental World is carried out using a dental microscope, which provides an enlarged image of the surgical field, this improves the quality of treatment and canal filling.
Devital extirpation
Previously, patients referred to this method as “putting arsenic.” The technique involves removing the pulp after it has been previously devitalized ( necrotization ) by applying medications. Devital extirpation is carried out if it is impossible to perform depulpation using the vital pulpectomy method. For pulpitis of permanent teeth with unformed roots, this method is not used.
To devitalize the pulp, arsenic acid paste is used. The paste also includes painkillers, antiseptics and anti-inflammatory agents, glucocorticoids and components that slow down the absorption of arsenic into tissues, since the substance is highly toxic to the body.
Arsenic paste is applied after removing softened dentin and opening the pulp. Arsenic paste is applied to single-rooted teeth for 24 hours, and to multi-rooted teeth for 48 hours.
A devitalizing agent with less toxic properties is paraformaldehyde paste. It causes pulp necrosis within a week in single-rooted teeth, and within 10-14 days in multi-rooted teeth.
Depulpation using the devital pulpectomy method is carried out in two visits:
- After treating the carious cavity, a devitalizing paste is applied to the pulp. A temporary filling material is placed.
- The temporary filling is removed, the tooth cavity is opened, and the pulp is removed. Next, the cavity is washed, dried, the root pulp is removed, the canals are treated with medications and filled - the same as with vital pulpectomy, but without the use of anesthesia, since after devitalization and death of the pulp, the tooth becomes insensitive to irritants.
Devital amputation
The devital amputation technique is used for complete obstruction of the canals. After applying the devitalizing composition, the necrotic coronal pulp is removed and the root pulp is impregnated using resorcinol-formalin paste or other mummifying compounds. After 2-3 times impregnation, the root pulp polymerizes and cannot rot.
This method is rarely used in clinical practice and, as a rule, in weakened patients who have suffered a myocardial infarction, stroke, or various severe operations. After removal of the devitalized coronal pulp, mummification of the root pulp is carried out using impregnation of resorcinol-formalin or any of the mummifying pastes.
The development of pulpitis can be prevented if you monitor the condition of the oral cavity and treat caries in a timely manner, since most often pulpitis develops as a consequence of a deep-seated carious process. A preventive examination by a dentist, which should be carried out twice a year, helps to detect caries at an early stage.
results
Statistical analysis of the results was performed using the Kruskal-Wallis test. There were no statistically significant differences between groups regarding the severity of pulp inflammation (H=0.679 with three degrees of freedom, P=1.00). However, in both the Portland cement group and the light-curing pulp capping group, cases of dentin bridge formation by day 28 (Table 2) were significantly more common than in the GIC and VLC Dycal groups (H= 11.989 with three degrees of freedom, P=0.009). Dentin bridge thicknesses in the Portland cement and light-cured pulp capping groups were also statistically greater than those in the other two groups (H=15.849 with three degrees of freedom, P=0.002). In addition, the number of cases of pulp necrosis in the GIC group was higher than in the other groups.
Table 2. Formation of a hard tissue barrier by day 28
| Presence of a barrier | TheraCal | Portland cement | GIC | Dycal |
| Yes | 11 | 12 | 4 | 4 |
| No | 1 | 0 | 8 | 8 |
The feasibility of direct pulp capping and the use of therapeutic pads
In modern dentistry, issues related to direct coating of the dental pulp and the use of therapeutic spacers to preserve the vitality of the pulp remain unclear and controversial. The results obtained by individual specialists are currently contradictory and are under intensive research and cannot yet serve as a basis for generalizing recommendations on this issue. Even experienced dentists sometimes experience great difficulty in choosing the optimal treatment methods for reversible pulpitis, drawing up a rational plan of treatment and preventive measures, including stopping the inflammatory process in the pulp, influencing the microflora, reducing pain and normalizing metabolic processes in the dental pulp.
Despite the rapid development of therapeutic dentistry and progress in endodontics, debate about the pros and cons of direct pulp capping and the use of therapeutic pads still continues throughout the world.
With this in mind, I have tried to bring some clarity to this controversial issue and express my opinion on the advisability of direct dental pulp capping and the use of therapeutic spacers to preserve the vitality of the pulp. Without claiming encyclopedic completeness, I tried to highlight a topic of interest to practicing doctors and answer those questions that, for one reason or another, usually “drop out” from monographs and journal articles.
First, a little about the “traditional” method of using medicinal pads in “classical” domestic dentistry, which we studied (and are studying) in institutes. Many textbooks on therapeutic dentistry recommend the use of calcium hydroxide preparations under a lining of zinc phosphate cement.
I considered it possible to only briefly touch upon the description of this “classical” technique, since, in my opinion, it is absurd. A doctor who has a basic understanding of the school chemistry curriculum will not place calcium hydroxide preparations under a zinc-phosphate cement lining, for one simple reason. Everyone knows that zinc phosphate cement has a sharply acidic pH, especially in the first days after mixing. The therapeutic effect of preparations based on calcium hydroxide is based on a sharply alkaline pH (12.5). When using this “classical” technique, a basic chemical reaction of neutralization occurs and the “therapeutic” pad, in the very first hours after application, turns into a useless mess with a neutral environment.
I also believe that it is inappropriate to leave any medications at all under permanent restoration for several reasons. Firstly, the duration of the therapeutic effect of any medicinal substance weakens over time, and eventually stops quite quickly. This raises the question - why is it under the “permanent” restoration that we are putting in place for at least several years? Secondly, any therapeutic pad contains active substances, which, while providing a therapeutic effect, greatly decrease in volume over time. In this regard, a void appears under the filling, which does not have the best effect on the prognosis of this restoration.
Therefore, my first recommendation: if you decide to install a therapeutic pad, then place it only under a temporary filling, for the time necessary for the expected therapeutic effect. Secondly, do not forget to consider the interaction of different materials.
So, when should you use medicated pads? It is extremely important for the doctor to determine which treatment is indicated - endodontic or prophylactic. The feasibility of using therapeutic pads primarily depends on the extent of the process in the pulp. Depending on the extent of the pathological process, we choose a treatment method, ranging from a sedative bandage to endodontic treatment.
Let me remind you that pulpitis can be reversible and irreversible. With any type of irreversible pulpitis, it is impossible to clinically determine the degree of inflammation of the pulp (partial or complete). According to modern concepts, any type of irreversible pulpitis requires immediate endodontic treatment, which aims to completely remove the infection from the canal and periapical tissues and ends with a complete, dense and hermetically sealed filling of the root canal system with non-irritating materials. In this case, medical pads are out of the question. If we have reversible pulpitis (in the domestic classification, this is deep caries), then to preserve the viability of the pulp, we can try to use various therapeutic pads.
If you nevertheless decide to install a therapeutic pad in order to preserve the viability of the pulp, then you need to imagine what material you need to use and what you want to achieve from its use.
All materials for applying therapeutic pads in order to preserve the viability of the pulp can be divided into two groups.
The first group consists of substances with a strong but short-term effect. Such drugs are applied for 1-3 days under a temporary filling. They are designed to quickly relieve inflammation of the pulp, relieve swelling, pain, normalize blood circulation in the pulp, and destroy pathogenic microflora. They contain strong anti-inflammatory, anesthetic and antibacterial drugs.
The second group is long-acting substances. They are applied for a long period of time - a month or more. Such drugs are intended to stimulate the formation of replacement dentin, normalize metabolic processes in the dental pulp, and prevent reinfection of the pulp. They include zinc oxide-deugenol paste, calcium hydroxide, hydroxyapatite, and weak long-acting antiseptics.
Materials for therapeutic pads should:
— have anti-inflammatory, antimicrobial, odontotropic effects;
- do not irritate the tooth pulp;
— ensure strong sealing of the underlying dentin, connection with tooth tissues, cushioning and permanent filling materials;
— get as close as possible to the physical and mechanical properties of permanent filling materials.
When treating reversible pulpitis, we must preserve the viability of the inflamed pulp and restore its functions. Treatment in such cases is carried out in two stages:
Stage 1 – aimed at stopping the inflammatory process in the pulp, influencing the microflora, reducing pain.
For this purpose, drugs that have a strong but short-term effect are used. They are usually applied for several days as a therapeutic dressing.
Stage 2 – aimed at stimulating the formation of replacement dentin and normalizing metabolic processes in the dental pulp.
At this stage, drugs are used that have a long-lasting, “soft” effect and do not decompose when left in the carious cavity for a long time. They are applied in the form of a therapeutic pad under temporary fillings, but for a long time.
Sometimes you can limit yourself to applying a therapeutic pad with a long-term odontotropic and antiseptic effect.
Depending on the composition, materials for medical pads are divided into 3 groups:
1. calcium hydroxide based materials,
2. zinc-eugenol cement (ZEC), 3. combined medicinal pastes. Calcium hydroxide based materials.
They are used most often. In our country, Calmecin is produced (contains calcium hydroxide, zinc oxide, sodium sulfacyl, dried blood plasma; mixed with a solution of carbomethylcellulose).
Among the foreign preparations containing calcium hydroxide as a base, the following should be mentioned: “Dycal” (DeTrey/Dentsply), “Calcium Hidroxyde XR” (SPAD/Dentsply), “Alkaliner” (Espe), “Septocalcine Ultra”, “Calcipulpe” ( Septodont), "Calcimol", "Calcimol LC" (Voco), "Life" (Kerr), "Reocap", "Reocap-E" (Vivadent), "Nu-Cap" (GC). These chemically or light-curing materials are the most versatile and most popular means of applying medicated pads.
One of the positive properties of these materials is their therapeutic odontotropic effect and the ability to prevent the penetration of microorganisms into the dental pulp, as well as rapid hardening, including in the presence of fluid released from the dentinal tubules.
However, the compressive strength of these materials is 10-15 times less than that of phosphate cement, and their stability in oral fluid is insufficient. Therefore, they should be applied only in a very thin layer to the areas of dentin closest to the pulp (indirect capping) or to the exposed pulp horn (direct capping), and topped with a layer of a stronger material, such as glass ionomer cement.
Some researchers are currently questioning the admissibility of direct application of calcium hydroxide-containing preparations to the exposed pulp due to possible osmotic injury to odontoblasts with the development of focal pulp necrosis.
Light-curing materials of this group are recommended for use only in shallow cavities due to the risk of thermal injury to the pulp during the process of light polymerization.
Zinc-eugenol cement.
Eugenol is an antiseptic of plant origin. It makes up 70% of clove oil. When mixing zinc oxide and eugenol, cement is formed that hardens within 10-12 hours. Curing is based on a chemical reaction that forms zinc eugenolate.
Preparations of this cement, which also contain strengthening substances, are more convenient for manipulation. Among the imported drugs supplied to the Russian market are (DeTrey/Dentsply), “Cavitec” (Kerr), “Zinoment” (Voco), “Cp-CAP” (also contains calcium hydroxide) (Lege Artis).
I would like to remind you that materials containing eugenol should not be used in combination with composites, as it disrupts the polymerization process of the organic matrix.
Combined medicinal pastes.
They include several groups of medicinal substances and are prepared ex tempore, taking into account the clinical situation, compatibility, availability in the medical institution and the individual preferences of the doctor.
The main groups of medicinal substances used in the preparation of combined medicinal pastes:
a) Odontotropic agents - substances that stimulate the formation of replacement dentin and remineralization processes in the zone of demineralized “carious” dentin: calcium hydroxide, fluorides, calcium glycerophosphate, dentinal or bone filings, hydroxyapatites (natural and artificial), “Algipor”, collagen, etc.
b) Anti-inflammatory drugs: glucocorticoids (prednisolone, hydrocortisone), less often - non-steroidal anti-inflammatory drugs (indomethacin, etc.).
c) Antimicrobial substances: chlorhexidine, metronidazole, lysozyme, sodium hypochlorite, etonium paste (7% etonium in artificial dentin). The advisability of including antibiotics in the composition of a therapeutic pad is currently controversial.
d) Proteolytic enzymes: profezyme, imozimaza, tomatozyme, especially in combination with other substances (chlorhexidine), sometimes prove to be quite effective in the treatment of reversible pulpitis.
Other agents: hyaluronidase, EDTA, dimexide (DMSO), kaolin, zinc oxide, novocaine, various oils (clove, sea buckthorn, first eucalyptus, oil solutions of vitamins, etc.).
Combined pastes, as a rule, do not harden, do not have sufficient mechanical strength, and lose their activity relatively quickly. Therefore, I would recommend their short-term use and subsequent replacement with zinc-eugenol cement or calcium hydroxide-based hardening material.
Direct pulp capping.
Direct pulp capping is a dressing applied to exposed pulp to preserve its vitality. Opening of the pulp can occur during the preparation of hard dental tissues or trauma, and its further condition will depend on the effectiveness of measures taken to prevent the entry of bacteria into it. If the pulp has been exposed as a result of a carious process, the pulp capping procedure is contraindicated. Infiltration of bacteria into the pulp is an irreversible process and therefore the only solution in this case is endodontic treatment.
When the pulp is exposed, it is very important to achieve hemostasis, for which the use of a weak solution of sodium hypochlorite (1% or less) is recommended. If bleeding does not stop within one minute, endodontic treatment is indicated.
Although there is ongoing debate regarding pulp preservation, most experts believe that calcium hydroxide is one of the best drugs for capping exposed pulp.
Initially, calcium hydroxide preparations were liquid calcium hydroxide pastes consisting of a mixture of calcium hydroxide and water. The paste was easier to work with if methylcellulose was added to it. In the early 1960s, calcium hydroxide cement was created that could cure to a solid state.
It should be noted that when creating calcium hydroxide cement, the manufacturer faced the difficult task of giving this material balanced properties - to have a sufficiently high solubility necessary to maintain its therapeutic effect, and at the same time have stable characteristics to resist dissolution under a temporary filling. However, there is still much debate in the literature on the question of whether pulp capping materials need to have properties that stimulate the formation of secondary dentin.
Discussions about the use of dentin adhesive materials as a preparation for direct pulp capping are even more controversial compared to the use of calcium hydroxide preparations for this purpose, and this issue is under intensive research. As noted by Stanley in 1998, recent research on pulp capping has been inconsistent and sometimes inaccurate, reducing practitioners' confidence in the use of this treatment. Therefore, additional research is required in order to draw a definite conclusion on this issue.
The question of the possibility of direct application of dentin adhesive using total etching of dentin remains unclear and controversial, and it requires additional research. Some authors have reported that some success has been achieved with direct pulp capping with such preparations without acid etching, or with adhesives that do not require this step (self-etching primers), although phosphoric acid can act as an effective hemostatic agent. However, the results obtained by individual researchers cannot yet serve as a basis for a general recommendation on this problem.
Many methods for preserving the viability of the exposed pulp, described in “modern” textbooks and manuals on endodontics, are controversial. It should also be taken into account that some generally accepted scientific theories are not substantiated by anything other than the confused explanations of the authors themselves. Meeting resistance from “authoritative” scientists, many researchers try not to risk their well-being and study only those phenomena that are traditionally studied at their department.
Doctors actively involved in scientific research in the field of preserving the viability of the exposed pulp generally say that after the treatment, “everything was so good” that the patients did not even show up for a follow-up examination. Comments on such studies, as they say, are unnecessary.
Based on my personal observation, I would like to note that direct covering of the pulp in the event of its accidental opening is effective only under sterile conditions, which is practically impossible due to the presence of infected dentin between the carious cavity and the tooth cavity, and can only be used in case of accidental opening of the cavity of an intact tooth. tooth, which calls into question the qualifications of the doctor who made such a mistake. The use of therapeutic pads, as a rule, also does not cause the expected therapeutic effect from them, due to the inability to control the lines of formation of coagulative pulp necrosis and the presence of infected pulp.
It is my opinion that successful endodontic treatment is much better than the unpredictable results of treating the exposed pulp by protecting it, which can ultimately lead to resorption, inflammation or necrosis. However, preserving the vitality of the exposed pulp may be advisable in young individuals to ensure normal development of tooth roots.
We must not deceive ourselves. Failures in maintaining the viability of the exposed pulp do occur and will continue to occur, despite the great efforts of doctors and the constant improvement of techniques. Our goals may be noble and lofty, but we cannot always achieve them, and this is often due to the fact that we are dealing with a human body that does not always behave as it is written in books.
I understand perfectly well that the topic raised in this article is currently controversial. My opinion on this issue is very different from the views accepted in the domestic scientific school. The article may contain controversial provisions, but, nevertheless, I express the hope that doctors will be able to use the above recommendations in their daily practice.
Clinical methods
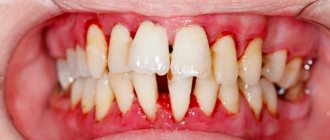
The patient came for planned treatment for recurrent caries at the border with an incompetent restoration occupying the chewing and lingual surfaces of the tooth. Before this appointment, he had no complaints of increased sensitivity or discomfort. After local anesthesia, a rubber dam was applied. After removal of the old restoration, microleakage of the filling and, as a consequence, resorption of the lining material was discovered. Further removal of the infected tooth tissue led to the opening of the pulp (Fig. 3). When the lingual fissure was stained with a caries detector, there were no signs of demineralization, so there was no need for its excision.
| Rice. 3. Pulp exposure in an asymptomatic molar. During the careful removal of carious dentin, one of the pulp horns was opened. Careful hemostasis must be performed before continuing treatment. |
Bleeding from the pulp tissue was stopped by applying a sterile cotton swab soaked in a solution of local anesthetic (Fig. 4). Other recommended solutions to stop bleeding are ferrous sulfate, sodium hypochlorite, 2% chlorhexidine (Cavity Cleanser, Bisco). Regardless of which solution is used, the clinician should be careful to ensure that it does not interfere with the adhesion of permanent restorative materials.
| Rice. 4. After the bleeding has stopped, it is clearly visible that the exposed area of the pulp is much smaller than it seemed at first |
When applying the new polymeric light-curing material (Table 3) to cover the TheraCal pulp, apply pressure to the syringe plunger for only 1-2 seconds. When the material begins to flow out of the syringe, apply a small ball of material that forms at the end of the needle to the bottom of the prepared cavity. The material is easily distributed with a needle and completely covers the exposed pulp area as well as approximately 1 mm of surrounding dentin. The applied layer should be less than 1 mm thick, ideally 0.3-0.5 mm. In Fig. Figure 5 shows that the pinkish pulp tissue is still visible through the layer of covering material. After application, TheraCal is polymerized for 20 seconds with a light flux density of at least 500 mW/cm2.
| Rice. 5. Application of light-curing pulp capping material |
After the material has polymerized, the doctor can begin traditional bonding and restoration procedures. In this case, the tooth was restored using a “total etching” technique and using a low-shrinkage composite material (Fig. 6). The prepared cavity was treated with 32% Semigel etchant with benzalkonium chloride (Bisco Inc, USA) for 30 s. The etching gel was applied first to the enamel and then to the dentin for 10 s. After rinsing the cavity, excess moisture was removed, leaving the dentin moist.
| Rice. 6. “Total etching” technique with 32% BAC gel applied first to the enamel and then spread onto the dentin. The cavity is washed abundantly with water. Excess moisture is carefully removed using a powerful vacuum cleaner, leaving the dentin moisturized. Two layers of One Step adhesive are then applied and light cured before filling the cavity with a modified GIC and low shrinkage composite material. |
Then two layers of One Step adhesive (Bisco Inc, USA) were applied and cured with light. The cavity was filled using the sandwich technique. The first layer of polymer-modified GIC was applied up to the enamel-dentin border. The tooth was then restored with packable composite material Aelite LS, which was applied first to the edges of the cavity and then reproduced the anatomical shape of the tooth (Fig. 7). Each layer of material was cured for 20 s with a light output of at least 500 mW, first on the buccal side, then on the lingual side, and finally on the surface. The last layer was smoothed using a Thomas float, then a layer of BisCover surface sealant was applied. After light curing of the sealant layer, the rubber dam was removed and the occlusion was checked. The surface can then be polished in the usual manner using carbide finishes and rubber heads.
| Rice. 7. Apply the last layer of Aesthetic LS, color A1, and form the anatomical structure of the tooth using a plugger. To improve the surface characteristics of the restoration, BisCover sealant was applied |
Follow-up is necessary in all cases where pulp viability may be compromised. The patient was warned to call immediately if any symptoms occurred, with particular attention to temperature sensitivity or discomfort when biting on the tooth. If these symptoms appear, an X-ray examination is indicated, and then, if necessary, endodontic treatment. The patient did not complain of any symptoms during the follow-up visit (Fig. 8).
| Rice. 8. Repeat visit 6 months after treatment. The patient does not complain of increased sensitivity after rehabilitation therapy |
Clinical case No. 2
Patient, 44 years old. During the planned replacement of a composite filling in tooth 17 and excision of necrotic dentin, the pulp was exposed. There were no signs of irreversible pulpitis. The pulp is vital, independent hemostasis within 40 seconds. At the same visit, direct pulp capping was performed. At the next visit - direct composite restoration of the tooth. At a follow-up examination 2 years later, there were no signs of irreversible pulpitis.
Rice. 13
Rice. 14
Rice. 15
Rice. 16
Rice. 17
Rice. 18
Discussion
Portland cement (MTA) fully satisfies the requirements for it in cases of treating teeth with exposed pulp. • Although both glass ionomer cement and light-curing polymers containing calcium hydroxide are also recommended for direct pulp capping, they are not the materials of choice. • On the other hand, MTA or pure Portland cement is difficult to apply and takes too long to set. • TheraCal is easy to apply and cures within 20 seconds of curing. TheraCal powder continues to harden due to the penetration of water into the hydrophilic polymer matrix, which increases the strength properties of the material. In addition, the alkaline pH and the presence of calcium oxide in its composition determine the biocompatibility and antibacterial properties of this material.
Table 3. Acidity level of different versions of TheraCal and Dycal VLC
| Day | TheraCal A | TheraCal B | TheraCal C | Dycal |
| 1st | 11,191 | 10,911 | 11,288 | 8,599 |
| 28th | 9,327 | 8,606 | 9,667 | 7,868 |
| 168th | 8,738 | 7,894 | 8,752 | 7,619 |
| 265th | 8,481 | on day 175 pH = 7 | 8,466 | on day 170 pH < 7 |
Light-curing TheraCal has found rapid clinical acceptance as a material with good adhesion to wet substrates. A small amount of material can be syringed into the deepest part of the cavity or applied to the exposed pulp. TheraCal, after polymerization, is firmly retained in the cavity and is not washed away during the etching and rinsing procedure. In addition, a very thin layer of material is sufficient to create the desired effect. Of course, the TheraCal layer should not be too thick, since in this case it will not completely polymerize. In addition, a thick layer can compromise the strength characteristics of the permanent restoration. In appearance, TheraCal material is similar to tooth enamel, so its use does not cause problems from an aesthetic point of view.
To date, none of the patients we have treated with TheraCal have complained of hypersensitivity or experienced complications. Of course, this information, based on anecdotal evidence, as well as our extensive clinical studies, needs to be confirmed by the long-term positive results of using this light-curing direct pulp capping material.
Clinical case No. 3
Patient, 22 years old. Extensive carious cavity. Despite the great depth of the lesion, there were no signs of irreversible pulpitis. The patient refused endodontic treatment. Alternatively, a decision was made to use a direct pulp capping technique. Indirect tooth restoration is recommended. At examination 2 years later, there were no signs of pulpitis. The vitality of the tooth is preserved. The patient did not complete the recommended treatment.
Rice. 19
Rice. 20
Rice. 21
Rice. 22
Rice. 23
Acknowledgments
The authors would like to express their appreciation for the invaluable assistance provided by Drs Arthur Weiss and Dr Stuart Stock, without whom this project would not have been possible. We would also like to thank Dr. Eugene Lautenschlager for the statistical analysis he performed. Materials for this project were donated by Bisco (Schaumburg, IL) and GC America (Alsip, IL).
©Based on materials from the scientific and practical journal “Clinical Endodontics”: volume II, No. 1-2, 2008
Indirect pulp capping
Indirect dental pulp capping can be done if the pulpitis is reversible. The tooth should have no symptoms of irreversible pulpitis (spontaneous pain, prolonged painful reactions to cold, inability to relieve pain when taking analgesics), there should be responses to tests showing the viability of the pulp (cold test, EDI) and no changes in the periapical tissues of the tooth.
Although the remaining dentin thickness between the pulp and the carious lesion cannot be accurately estimated, at least 0.5 mm of uninfected dentin is necessary. When dentin thickness is less than 0.5 mm, pathological changes may develop in the pulp.14
The purpose of indirect pulp capping is to prevent bacteria from accessing the nutrient substrate, thereby slowing down the progression of the caries process. The success of this method was described in one study.15 In this study, after preparing the cavity and collecting material for bacterial culture, a decrease in the formation of bacterial colonies was observed.16 One of the well-studied properties of MTA is the provision of a stable barrier to prevent further development of the caries process.10
Indirect pulp capping is carried out after partial extraction, when only carious infected dentin is removed, or after removal of both infected and caries-damaged dentin. Although one of the benefits of partial removal (step-by-step removal) is the prevention of pulp exposure, practitioners also need to be trained to handle accidental pulp exposure.
Clinical case 1: Partial removal of affected tissue
Girl, 6 years old, examination revealed 4 deep carious cavities on the first permanent molars. The patient noted a positive reaction to cold for several weeks. At the time of the examination, no complaints of spontaneous pain were detected.
The radiograph determined the stage of the unformed root and the absence of pathological changes in the periapical region. Anesthesia was performed and teeth were isolated using a rubber dam. Preparing the cavity to the enamel-dentin border, towards the pulp. During preparation, dense pigmented dentin is left in order to possibly expose the pulp.
Restoration was carried out using Filtek composite in color A2B. However, the treatment provided did not involve re-preparation of the teeth until, as one of the options, the restoration became untenable due to a seal failure, or until any symptoms appeared. During preventive examinations, the first permanent molars were asymptomatic; spot photographs did not reveal any periapical changes. I note that if dense pigmented dentin is left in the cavity, an area of clearing may be observed on the radiograph, therefore, when treating deep caries, it is necessary to take into account the results of both objective and additional research methods. It is also recommended to leave colored glass ionomer cement of the Fuji Triage type at the bottom of the formed cavity, as a warning that the affected tissue has not been completely removed.
Clinical case 2: Complete removal of affected tissue
A 17-year-old patient complained of pain in the upper jaw. The patient associated the pain with tooth 2.6. Upon examination, there is a large carious cavity on the chewing surface; probing the bottom is painful. The patient complained of pain when biting. Anesthesia was performed and a rubber dam was installed. The preparation was performed without opening the pulp; NeoMTA (NuSmile) was applied in the area of projection of the pulp horns using an amalgam tray and a cotton ball. The tooth was restored with composite material.
Bioceramics in endodontics
The main cause of apical and periradicular periodontitis is microbial invasion into the postapical space. Thus, the goal of endodontic treatment is to prevent microbial contamination in the root canal system, as well as to cleanse its space of existing microorganisms. Quality control of endodontic intervention is carried out according to clinical examination and x-ray diagnostics. A common misconception is that endodontic treatment involves intervention in the canal structure, possible re-intervention, and, if the previous two are unsuccessful, an apical surgery procedure. But preserving the vitality of the pulp is also endodontics, because a healthy pulp is the key to a healthy periodontal condition. Thus, pulp capping and pulpotomy are integral parts of endodontic practice. Root canal treatment includes two phases: the microbial contamination control phase (instrumentation, irrigation, medication administration) and the restoration phase (filling the endodontic space). In endodontics, there is only one type of material that is ideal for use in both of these phases - bioceramics, which has already been successfully used in dentistry for many years.
Definition of Bioceramics
Bioceramics are a subtype of ceramic materials that are developed specifically for medical and dental purposes. It consists of aluminum and zirconium, bioactive glass, coatings and composites, hydroxyapatite, resorbable calcium phosphate and radiopaque glass particles. Bioceramics are widely used in joint reconstruction, for implant biocoatings, and as resorbable membranes that maintain a certain amount of required space over a limited period of time.
Bioceramics are classified into:
- bioinert – does not interact with biological systems;
- bioactive – persistent in tissues, which is characterized by the presence of interactions at the interface with surrounding tissues;
- bioresorbable - soluble or absorbable, which is ultimately incorporated into the tissue structure.
Currently, there are many representatives of bioceramics that are used in dentistry and medicine. Ceramics based on aluminum and zirconium are bioinert and are used in orthopedics. Bioactive glass ceramics are available in dentistry under a variety of brand names, and calcium phosphate-type porous ceramics are often used to repair bone defects. Certain representatives of bioceramics, such as those based on calcium silicate (mineral trioxide aggregate [MTA], ProRoot MTA Root Repair, Dentsply Sirona) or based on a bioaggregate (DiaRoot BioAggregate, DiaDent) have also been used in dentistry as materials for restoring defects in the root structure and for apical execution of the endodontic space.
Properties of endodontic bioceramics
Endodontic bioceramics is not sensitive to moisture and blood contamination, that is, the quality of its use does not depend on the technique of performing the procedure. It is also dimensionally stable and expands somewhat as it hardens. In its stable state, bioceramics are very hard and perfectly compacted, but do not dissolve over a long period of time, thus ensuring the prevention of microleakage. During hardening, the pH of the material rises to 12 as a result of a hydration reaction. In this process, calcium hydroxide is first formed, which is then dissolved into calcium and hydroxyl ions. The high pH of the material during hardening ensures its antibacterial properties. In a stable state, the material is not only completely biocompatible, but also bioactive. When bioceramics come into contact with surrounding liquids, calcium hydroxide is released, which can interact with phosphates in the liquid and ensure the formation of hydroxyapatite. These material properties can be categorized as inductive with respect to surrounding hard tissue. Considering all this, it is clear that bioceramics are the material of choice for pulp capping, filling spaces after pulpotomy procedures, restoring root defects resulting from perforations, filling endodontic spaces and obturing teeth with immature roots to induce the process of apexification.
Available bioceramics in endodontics
MTA
Few clinicians understand that the original MTA is a classic bioceramic material with the addition of some heavy metals. It has all the properties of bioceramics, including high curing pH, biocompatibility and bioactivity, as well as ideal compaction parameters. However, the material also has some disadvantages. Hardening time is about 3 hours. During its preparation, the material must be kneaded, which increases its consumption. Both gray and white MTA stain dentin, apparently due to the presence of heavy metals in their structure, or due to the inclusion of pigments from the blood during the hardening process. MTA is also quite difficult to introduce into narrow channels. To correct all these shortcomings, MTA is constantly modified, but any changes also affect its initial almost ideal chemical and physical characteristics.
Biodentine
Biodentine (Septodont) is considered a representative of the second generation of bioceramics. Its characteristics are similar to MTA, and thus it can be used for all the same indications as MTA. However, unlike MTA, it hardens much faster (10-12 minutes), and its density is closer to that of dentin. The disadvantage of the material is the form of its delivery: delivery of biodentine is possible only for 30 seconds from the capsule, and as a rule, the volume used is in most cases less than that contained in the capsule
Pre-mixed bioceramics
Premixed bioceramics are available only in North America and include EndoSequence BC Sealer, EndoSequence BC RRM (Root Repair Material, syringe paste form), and EndoSequence BC RRM-Fast Set Putty. All three of these materials are similar in chemical composition (calcium silicate, zirconium oxide, tantalum oxide, monobasic calcium phosphates and fillers), and have excellent mechanical and biological properties, as well as being fairly easy to work with. They are hydrophilic, insoluble, radiopaque, aluminum-free, high pH and require moisture to cure. The working time of BC Sealer and BC RRM is over 30 minutes and the curing time under normal conditions is approximately 4 hours, depending on the amount of moisture available. Recently introduced to the market, EndoSequence BC RRM Fast-Set Putty has all the properties described above, but features a shorter cure time (approximately 20 minutes). This material is suitable for perforation closure, retrograde filling and vital pulp capping. BC Sealer is essentially the only pure bioceramic material that is available specifically in the form of an endodontic sealer. Its chemical composition is the same as other pre-mixed bioceramics, but it is less viscous, making it an ideal consistency for compaction in root canals. It can be used with gutta-percha points impregnated with a layer of bioceramic nanoparticles. In this case, gutta-percha also plays the role of a sealer carrier (photos 1 - 3), so BC Sealer can reach the tubule system in the apical part of the root (photos 4 - 5).
Photo 1. Filling the endospace with bioceramics: cut at a distance of 0.5 mm from the apex.
Photo 2. Filling the endospace with bioceramics: cut at a distance of 1.5 mm from the apex.
Photo 3. Filling the endospace with bioceramics: cut at a distance of 3.0 mm from the apex.
Photo 4. X-ray of the tooth before filling the root with bioceramics.
Photo 5. X-ray of a tooth after filling the root with bioceramics.
The connection of these gutta-percha points and the bioceramic sealer ensures maximum sealing of the endospace, thus minimizing the risk of developing potential microleakage. If repeated treatment is necessary, the same gutta-percha serves as a guide for the passage of the canal. To date, more than 50 studies have been conducted to prove the effectiveness of using pre-mixed bioceramics. A recent study comparing the results of apicoectomy with MTA retrograde filling and a bioceramic analogue in dogs showed that bioceramics achieved a slightly better outcome of the procedure, apparently due to its adapted manipulation properties.
Indications and examples of clinical cases
Direct and indirect pulp capping
Historically, endodontists initially did not recommend performing any manipulations in the area of the intact pulp of carious teeth. But studies conducted in the 1970s showed that something still needs to be done with the pulp in deep carious cavities. The first results of such interventions were not very successful, since after covering the pulp with calcium, doctors used amalgam as the main restorative material for the coronal part. It is logical that microleakages that developed at the interface between the amalgam and the tooth also negatively affected the calcium-containing lining. In some cases, doctors noted the effect of canal calcification, in others – pulp necrosis and the development of periodontitis. One thing was clear: something had to change. New research and case series have shown that if the base used is antibacterial (eg calcium hydroxide) and, once cured, provides good compaction and stability, the prognosis for such intervention is good, regardless of whether the pulp is covered directly or indirectly. In young people, this type of therapy should be considered the treatment of choice.
Case 1: Direct pulp capping
Photo 6 shows a pre-treatment radiograph of tooth No. 19 with a carious lesion in a 20-year-old patient. The diagnosis of reversible pulpitis was made based on an analysis of the anamnesis and clinical examination data. After anesthesia and preparation, pulp exposure was noted (photo 7), the area of which was covered with BC RRM-Fast Se (photo 8). After complete curing of BC RRM-Fast Se, the defect was restored with a composite and control radiography was performed (photo 9). After 6 months, the tooth did not show any symptoms and, according to diagnostic data, remained vital. There were no radiological signs of pathology (photo 10).
Photo 6. X-ray of the 19th tooth with significant carious lesions.
Photo 7. View of the tooth after removal of the carious lesion.
Photo 8. Direct pulp capping with BC Putty bioceramics.
Photo 9. X-ray of the tooth after covering the pulp with bioceramics.
Photo 10. X-ray of a tooth six months after covering the pulp with bioceramics.
Clinical case 2: Pulpotomy
In this case, the tooth was vital during diagnosis, but showed signs of irreversible pulpitis. Treatment with a complete pulpotomy was chosen to improve the prognosis of the functioning of the remaining pulp. On the x-ray before treatment, a carious lesion can be detected in the area of the 11th tooth, as well as a slight expansion of the periodontal ligament. A complete pulpotomy was performed using BC putty (Figure 12). After curing of the material, a composite restoration and a control radiograph were performed. One year after treatment, the tooth was asymptomatic and the radiograph showed evidence of continued root development (Figure 13) in the presence of healthy periodontal tissue. There were no traces of calcification in the residual part of the pulp (these often form when calcium hydroxide is used). A radiograph taken of the opposite tooth showed a similar degree of root development (Figure 14).
Photo 11. X-ray of the tooth before treatment.
Photo 12. X-ray of the tooth after pulpotomy.
Photo 13. X-ray of the tooth 1 year after pulpotomy.
Photo 14. View of the tooth from the opposite side (for comparison).
Clinical case 3: Apicoectomy and retrograde filling
The patient sought help with clinical symptoms and radiographic signs of post-endodontic disorders (Figure 15). It was determined that protrusion in the mesial canal area interfered with the favorable prognosis of repeat endodontic treatment, and thus the decision was made to perform an apicoectomy procedure. During the intervention, an isthmus was discovered between the two main canals of the tooth (photo 16). Retrograde filling was performed both in the area of the main canals and in the isthmus area using BC RRM-Putty (photo 17). During the 20-month monitoring, no abnormalities in the treated tooth could be recorded (photo 18).
Photo 15. X-ray before treatment.
Photo 16. View after apicoectomy.
Photo 17. Filling of the isthmus after apicoectomy.
Photo 18. X-ray 20 months after filling the retrospace with BC Putty.
Root canal obturation
Historically, root canal obturation is the least predictable endodontic intervention. Doctors typically use fillers (silver or gutta-percha points) and sealers for this procedure to help achieve proper sealing. Traditional sealants shrink in volume as they cure and wash out over time. Some of their representatives are characterized by the presence of adhesion to dentin, but not to filler. To minimize this effect and reduce the risk of microgap formation, approaches to canal obturation have continued to be modified. For this purpose, cold lateral and hot vertical condensation methods have been developed. But at the same time, lateral cold condensation of gutta-percha is characterized by excessive disposition of the sealer in the area of the accessory tubules, while for hot vertical condensation it is necessary to remove an excessive amount of coronal dentin to advance the plugger tip to a distance of 4 mm from the apex. In addition, when gutta-percha is heated and cooled, it shrinks even more than conventional sealant. All this increases the risk of repeated bacterial contamination.
BC Sealer is a pre-mixed bioceramic product with excellent consistency for use as a sealer. Its pH level is as high as possible, it requires moisture to cure, and its dimensional stability is enviable - what else do you need for a good endodontic sealant? BC Sealer has not been proven to have any negative effects from blood or inflammatory exudate, allowing its use in a variety of clinical settings. Given the stability of ceramics, it is possible to use it in thick layers without fear of washing out and shrinkage. The filler in the bioceramic system is used only as a pump to deliver the filler to the apex area. In addition, the installation of bioceramics does not require excessive removal of dentin tissue, and such an intervention is, in fact, minimally invasive. An additional advantage of the system is that now special gutta-percha points have been developed for bioceramics, impregnated with nanoparticles of the same bioceramics, thus ensuring their reliable connection (photos 19 - 21).
Photo 19. X-ray before treatment: apical periodontitis.
Photo 20. X-ray after treatment.
Photo 21. View 5 years after treatment.
Authors: Martin Trope, DMD Gilberto Debelian, DMD, PhD