Where is euthanasia legalized?
Currently, euthanasia is officially permitted in the following states:
- Netherlands. The law was adopted in 2002. At the same time, a list of conditions was approved that are a significant justification for making this decision. Moreover, each case of euthanasia is reviewed by a special “ethics committee”, which approves or rejects the procedure.
- Belgium. The country also adopted a law in 2002, following the Netherlands. Its provisions apply only to citizens of the state permanently residing in Belgium. The basis for euthanasia is unbearable physical suffering that cannot be alleviated by existing medications.
- Switzerland. In this country, the procedure is allowed to be carried out not only by citizens, but also by foreigners who have expressed their will to die. Special clinics have been created in the state to carry out euthanasia.
- USA. The procedure is not legalized throughout the country. Oregon was the first state to allow euthanasia. Then Washington followed suit, and then Montana. Several years ago, “artificial death” was allowed in seven more states, including California, Hawaii, New Jersey and some others.
- Canada. By law, euthanasia can be carried out only by citizens of the state who have reached the age of majority. The procedure is not performed for foreigners. Most of the country's population actively supports allowing euthanasia, which can help sick people stop suffering and die peacefully. Canadians believe that death must be “justified and foreseeable.”
- Mexico. In this country, euthanasia will be allowed in 2022. Then the “Law on Death with Dignity” was adopted and conditions were approved in which the procedure would be justified.
In 2022, New Zealand held a referendum and vote to legalize euthanasia. The initiative was supported by more than 60% of the country's residents. The law is currently under development and will come into force very soon.
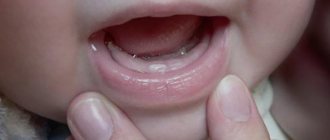
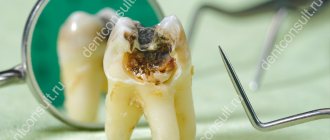
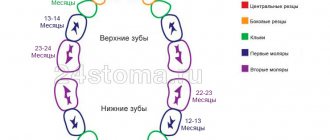
Pain-relieving teething gels can be hazardous to children's health.
“Children’s gels based on lidocaine are dangerous due to the classic side effects of lidocaine, which, in addition to its local analgesic effect, is also an antiarrhythmic - a drug for the treatment of certain heart rhythm disorders. In addition, when using lidocaine-containing gels in children, other undesirable effects were observed: convulsions, often leading to traumatic brain injuries, breathing problems, swallowing, and - the most serious complications - heart rhythm disturbances and death.
Strictly speaking, such gels are ineffective. The effect of local anesthetic drugs is due to their local action; in the oral cavity they are washed off with saliva and swallowed by the child, which leads to a very short-term analgesic effect.
It should be borne in mind that the form of the gel is not very convenient for use: not all parents read the instructions on the dosage of the gel in millimeters, frequency of use, etc. That is, the danger of an overdose of such gels is quite high. It is generally quite difficult to predict the proarrhythmogenic effect of a drug. Accordingly, the Food and Drug Administration (FDA), in its ban, was guided by the balance of harm and benefit of this drug. Roughly speaking, we do not expect serious complications from teething pain, so the use of a drug that can lead to the above complications and even death seems unreasonable.”
Pediatrician
Natalya Vasilyeva
——————————————————————————————————————————————
“I want to say right away: in our practice we have not used gels with lidocaine for a long time. Back in June 2014, the website for doctors medscape.com published information about their dangerous side effects. Gels with lidocaine in overdose can cause cardiac conduction disturbances, even leading to cardiac arrest in a child. Another serious side effect is seizures. There is also evidence that lidocaine gels can affect the composition of hemoglobin and the ability of blood to carry oxygen. An overdose can happen very easily. The fact is that all these gels give a very brief and insignificant effect, easing the child’s condition for just a couple of minutes. Because of this, parents have to smear the child’s gums with gel again and again, and this is very dangerous.
Pediatrician at the Fantasy Clinic,
Candidate of Medical Sciences
Ruzanna Avanesyan
How the procedure works
In order for euthanasia to be approved, it is necessary to write an application, perhaps even more than one. This is done in order to confirm the patient’s sincere desire to take such a serious step. A special commission, which must include doctors, psychologists and lawyers, considers the indications for the procedure, studies the patient’s medical history and makes a final decision.
The commission members also evaluate the patient’s ability to make independent, informed decisions. The attending physician must make sure that the pathology is indeed incurable and there are no methods that can alleviate the patient’s condition.
If the patient does not change his decision during the inspection, and the commission finds compelling reasons to approve euthanasia, the preparation of documentation and the selection of medications begins. The procedure can only be performed by a doctor.
First, the patient is administered strong narcotic analgesics, and then drugs intended directly for euthanasia. They are made on the basis of barbiturate. The medication is administered into the patient's body parenterally.
Lidocaine Velpharm injection solution 2% 2 ml 10 pcs
When used simultaneously with barbiturates (including phenobarbital), it is possible to increase the metabolism of lidocaine in the liver, reduce the concentration in the blood plasma and, as a result, reduce its therapeutic effectiveness.
When used simultaneously with beta-blockers (including propranolol, nadolol), the effects of lidocaine (including toxic ones) may be enhanced, apparently due to a slowdown in its metabolism in the liver.
When used simultaneously with MAO inhibitors, the local anesthetic effect of lidocaine may be enhanced.
When used simultaneously with drugs that block neuromuscular transmission (including suxamethonium chloride), the effect of drugs that block neuromuscular transmission may be enhanced.
When used simultaneously with hypnotics and sedatives, the inhibitory effect on the central nervous system may be enhanced; with ajmaline, quinidine, the cardiodepressive effect may be enhanced; with amiodarone, cases of the development of seizures and CVS have been described.
When used simultaneously with hexenal, sodium thiopental (iv), respiratory depression is possible.
When used simultaneously with mexiletine, the toxicity of lidocaine increases, with midazolam - a moderate decrease in the concentration of lidocaine in the blood plasma, with morphine - an increase in the analgesic effect of morphine.
When used simultaneously with prenylamine, there is a risk of developing ventricular arrhythmias.
Cases of agitation and hallucinations have been described when used simultaneously with procainamide.
When used simultaneously with propafenone, the duration and severity of side effects from the central nervous system may increase.
It is believed that under the influence of rifampicin, a decrease in the concentration of lidocaine in the blood plasma is possible.
With simultaneous intravenous infusion of lidocaine and phenytoin, side effects of central origin may increase; a case of sinoatrial blockade due to the additive cardiodepressive effect of lidocaine and phenytoin is described.
In patients receiving phenytoin as an anticonvulsant, a decrease in the concentration of lidocaine in the blood plasma is possible, which is due to the induction of microsomal liver enzymes under the influence of phenytoin.
When used simultaneously with cimetidine, the clearance of lidocaine moderately decreases and its concentration in the blood plasma increases, and there is a risk of increased side effects of lidocaine.
Storage conditions for Lidocaine in pharmacies and clinics
The medicinal product intended for further sale is stored in special ventilated warehouses at a temperature not exceeding 25 degrees.
In medical institutions, the medicine is stored in a special closed box in the closet. If the room temperature is higher than the maximum permissible, then the drug is placed for storage in a refrigerator with a temperature of 12 degrees above zero.
Defective medicine must be disposed of in accordance with:
- SanPin 2.1.7.2790 – 10;
- by order of the Ministry of Health of the Russian Federation No. 382 dated December 15, 2002;
- Clause 1 of Article 31 and Clause 3. Art. 31 No. 86 – Federal Law “On Medicines”.
Expired medical medicine is delivered to the disposal site in a closed vehicle in special sealed containers or boxes. The destruction process, depending on the release form, may be as follows:
- Neutralization by heat treatment;
- Shredding using a shredder.
- Burying or burning.
Most often, this anesthetic is destroyed by burning or instillation. The sale or use of defective medicines is prohibited.
Features of pain relief
Damage to soft tissues from a burn varies in degree. But pain sensations do not always correspond to their level.
The first and second stages affect the superficial layers of the skin. In this case, the nerve endings are injured. They react to a violation of tissue integrity, causing constant excruciating pain.
With deeper injuries (grade 3-4), the damage can reach the bones. The tissues practically burn out and along with them the nerve cells are destroyed. For this reason, a person who has received severe injuries experiences pain when exposed to high temperatures, and then its intensity decreases significantly . After some time, the stage of active regeneration begins, inflammatory processes occur and painful symptoms return. Consequently, with deep burns, the victim rarely needs pain relief. The exception is receiving injuries of varying degrees.
To alleviate the condition of the injured person is one of the main principles of first aid. But this must be done correctly, using special means, otherwise, as a result of illiterate therapy, complications, inflammation and infection develop.
Before taking any action, you should determine:
- source of damage
- degree of damage,
- characteristics of injuries and their location,
- general condition of the victim,
- make sure there are no allergies to the drugs used.
The main first aid methods for burns include:
- cooling the injured area,
- anesthesia,
- disinfection,
- in case of urgent need, apply a transport gel bandage or film.
Assistance to patients with deep injuries should be provided by a qualified specialist in a medical facility. The use of home remedies in this case is unacceptable.
Shelf life of Lidocaine
Like any other anesthetic, it has an expiration date. Regardless of the release form, it is always indicated on the original cardboard packaging. Most often you can see it on the front or side of it.
The drug goes on sale in several varieties:
- Solution for injection and intravenous administration. Shelf life 5 years
- Gel . Maximum storage time 3 years.
- Ointment . Maximum storage time 3 years.
- Eye drops . Shelf life: 2 years.
- The spray can be stored for 3 years.
Important! The drug belongs to list B and does not require special storage conditions in the refrigerator.
All forms of the drug can be used during the entire shelf life, even if no more than 5 days remain until its effect expires. During this entire period, Lidocaine completely retains all its qualities.
It is unacceptable to use defective or spoiled Lidocaine . Before use, you must examine the packaging for damage and also check the expiration date. If the medicine has an unpleasant foreign odor rather than a menthol aroma, a heterogeneous consistency and a cloudy color, it should not be used.
Attention! After opening the package, the shelf life of Lidocaine changes:
- ampoules with solution should not be stored open;
- drops can be used for one month;
- gel, spray and ointment can be used for one year.
The use of lidocaine during this time is allowed only if storage conditions are met.