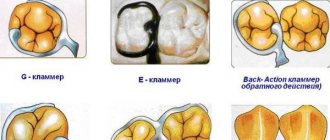
Dental stainless steel alloy - dental steel
Steel is the most common alloy in the world.
Its properties are well known. And due to alloying agents, it can be given any desired properties. Dental steel is very cheap.
Among the disadvantages: steel is heavy (density about 8 g/cm3) and chemically active. May cause allergies, galvanosis.
Stainless steel in orthopedic dentistry - grades:
STEEL GRADE 1X18H9T (EYA-1)
Dental alloy for crowns COMPOSITION :
1.1% carbon; 9% nickel;18% chromium; 2% manganese, 0.35% titanium, 1.0% silicon, the rest is iron.
Used for fixed prostheses: individual crowns, cast teeth, facets.
STEEL GRADE 20Х18Н9Т
COMPOSITION: 0.20% carbon, 9% nickel, 18% chromium, 2.0% manganese, 1.0% titanium, 1.0% silicon, the rest is iron.
The following are produced from this type of steel in the factory:
· standard sleeves used for the production of stamped crowns;
· clasp blanks (for ChSPP)
· elastic metal matrices for filling, as well as separation strips
· STEEL for dentistry GRADE 25Х18Н102С
COMPOSITION: 0.25% carbon, 10.0% nickel, 18.0% chromium, 2.0% manganese, 1.8% silicon, the rest is iron.
APPLICATION: manufactured in the factory:
· teeth (lateral upper and lower) for stamped-soldered bridges;
· frames for metal-plastic bridges, for cladding;
· orthodontic wire with a diameter from 0.6 to 2.0 mm (step 0.2 mm)
Silver solder is used as solder for non-precious alloys
Contains silver - 37%, copper - 50%, Manganese - 8-9%, Zinc - 5-6%
Source
Alloys in orthopedic dentistry
Metal occupies a central place among materials in dentistry. Most fixed dentures and removable denture frames are cast (or stamped) from dental alloys. Alloys in dentistry are used as auxiliary materials for soldering and stamping. Dental instruments are made from them.
- Classification of metals and alloys in dentistry
- Structural metal alloys in orthopedic dentistry
- Noble metal alloys in dentistry
- Base alloys in orthopedic dentistry
- Auxiliary metal alloys in dentistry
Primary requirements
The important properties that metals and alloys in dentistry must have are:
- high biological compatibility;
- resistance to corrosion and tarnishing;
- maintaining constant volume and shape;
- safety, no toxicity;
- inertness in the oral cavity;
- mechanical properties (strength, hardness, rigidity, wear resistance, ductility);
- physical properties (density, malleability, thermal conductivity, melting point);
- aesthetic indicators;
- hygienic properties (ease of cleaning with standard dental care products; in the case of using metal dentures, patients should not feel a metallic taste);
- technological characteristics (ease of preparation and processing, segregation).
This is interesting: Popular impression materials and their classification in orthopedic dentistry
Alloys that are intended for ceramic coating must meet the following criteria:
- connects well with porcelain;
- the temperature when processing the alloy should be higher than the temperature when processing porcelain;
- the thermal expansion value of the alloy should be equal to the CTE of porcelain.
An important aspect is shrinkage, which occurs during the transformation of the liquid state of the metal into a solid state.
If the shrinkage is too high, it makes the fabrication of dental structures difficult. Ease of metal casting is important to every dental technician. The lower the temperature when melting and pouring, the easier it is to work with them.
The characteristics of metals must necessarily meet the requirements associated with their purpose and processing methods.
Metals and alloys in dentistry Classification
All metals and alloys are divided into ferrous and non-ferrous.
Ferrous metals are iron and alloys based on it. Steel and cast iron. Cast iron contains more than 2.14% carbon. Not used in dentistry.
The surface of cast iron is matte and non-shiny. It is difficult to polish.
Steel in dentistry
an iron-based alloy containing less than 2.14% carbon. In addition to iron and carbon, steel also contains other metals. They give the alloy new properties (alloy steel), including making it stainless.
Steel copings for stamping crowns
Alloy steel is an alloy of iron and carbon, with the addition of any other metals. They change the properties of the alloy (melting point, hardness, ductility, malleability, etc.).
Alloy steel
Stainless steel is corrosion resistant steel. Chromium (21%), as well as other metals, are most often used as an anti-corrosion agent.
Non-ferrous metals are, respectively, all other metals.
Metals in orthopedic dentistry are divided into noble and non-noble.
Noble metals (or precious metals) are metals that are resistant to corrosion and chemically inert. The main noble metals are gold, silver, and platinum group metals (platinum, palladium, iridium, osmium, etc.).
Base metals are metals that corrode easily and are not found in nature in their pure form. They are always mined from ores.
Stainless steels
Stainless steels are steels that are resistant to corrosion in humid air, alkalis, salt solutions and weak acid solutions.
Stainless acid-resistant and heat-resistant steels are highly alloyed alloys of iron with chromium, nickel and a number of other additives (silicon, titanium, niobium, cobalt). The main elements that give these steels anti-corrosion properties are chromium and nickel. In accordance with the introduction of a particular metal into the alloy, steels are divided into chromium and chromium-nickel.
Chromium-nickel steels turned out to be more corrosion-resistant and are widely used in various branches of technology and in medicine.
Currently, there are several hundred grades of stainless steels for a wide variety of purposes.
In dental and general orthopedics, steel grades EYA1-T and EI-95 are used; they contain 17-20% chromium, 8-10% nickel and a small amount of carbon.
The resistance of stainless steels against corrosion is due to the introduction of chromium and nickel into them.
When more than 12% chromium is added to steel, the potential of the steel sharply improves and reaches a value of + 0.2, i.e., it approaches the potential of chromium in a passive state.
Chromium, which has the ability to passivate in the presence of oxygen, transfers this ability to steel, on the surface of which a protective film is formed as a result of the chemical combination of oxygen with chromium. An increase in the amount of carbon in steel leads to the formation of chromium carbides and depletion of the alloy in chromium, as a result of which the corrosion resistance of steel decreases. iChrome, by forming extremely hard carbides in the alloy, increases the hardness, tensile strength and elasticity of steel.
The addition of nickel to steel in an amount of up to 25% increases the hardness, tensile strength and yield strength of the steel, making it more tough, increases elongation and creates resistance to overheating of the steel during melting. The addition of nickel also greatly increases the corrosion resistance of steel in solutions of table salt, sulfuric and hydrochloric acids.
The introduction of other additives into steel, for example, titanium (steel grade EYA1-T), makes it insensitive to intergranular corrosion, increases resistance to tearing and impact, and reduces work hardening, which increases the ability of the steel to be stamped.
By introducing 2.5% silicon, it was possible to increase the heat resistance and fluidity of steel and obtain an alloy with good casting properties (steel grade EI-95, proposed by D. N. Tsitrin).
Stainless steels have a silvery-white color with a bluish tint and a bright metallic luster. Their specific gravity is 7.9, melting point is 1,400-1,425°.
The chemical composition and mechanical properties of stainless steels are given in the table.
A characteristic feature of chromium-nickel steels is their high plastic properties with moderate hardness and sufficient strength. Steel acquires its greatest ductility after heating to high temperatures (1,050–1,100°C) followed by rapid cooling. The mechanical properties of steel change dramatically after cold deformation and cold hardening. When compressing sheet steel, the tensile strength increases from 20-25 to 100 kg/mm2.
In terms of mechanical properties, stainless steels are not inferior to gold alloys. Steel is much harder than gold, so it is subject to less abrasion. Steel can be easily soldered with special solder and can be stamped relatively easily; after polishing it takes on a bright metallic shine with a silvery tint.
Below are comparative data on the mechanical properties of gold and chromium-nickel steel.
Corrosive properties. Chromium-nickel steels are completely resistant to corrosion in atmospheric conditions, in fresh and sea water, in all organic acids and alkalis.
In sulfuric acid, stainless steel is stable only at room temperature, in hydrochloric acid - only at low concentrations and a temperature of 20°. Stainless steel is quite stable in oral fluid. The corrosion resistance of steels decreases with increasing carbon content and decreasing chromium content; it can be increased by heat treatment and giving the steel an austenitic structure (a solid solution of carbon in iron).
The corrosion resistance of steel is affected by cold deformation (stamping of crowns), accompanied by the decomposition of the solid solution with the formation of carbides, and the quality of the surface finish of the dentures (grinding and polishing), since the condition of the surface affects the formation of a passivating film. With increasing grinding quality, the corrosion resistance of steel increases significantly.
Stainless steels are used in orthopedic dentistry for the manufacture of all types of dental and maxillofacial prostheses. Crowns, clasps, and orthodontic devices are made from steel grade EYA1-T. Steel grade EI-95, which has good casting properties, is used for casting teeth, facets, clasps and other parts of dentures.
Noble metals in dentistry and alloys
Noble metals in dentistry are expensive. But despite this, they continue to be used due to their excellent biocompatibility. They are not subject to corrosion, do not react with saliva, and do not cause allergies or intoxication.
A gold alloy may often be the only option for patients with polyetiological contact allergies.
Noble alloys are durable. Their only drawback (besides the price) is their softness and susceptibility to abrasion.
Gold alloys in dentistry.
COMPOSITION: 90% gold, 4% silver, 6% copper.
PROPERTIES: melting point 1063°C.
The alloy is characterized by ductility and can be easily machined under pressure (stamping, rolling, forging).
Due to its low hardness, the alloy wears off easily. Therefore, when making stamped crowns, solder is poured from the inside, onto the chewing surface or cutting edge.
Produced: in the form of disks with a diameter of 18, 20, 23, 25 mm and blocks of 5 g.
Application: for stamped crowns and bridges made of
alloy of precious metals in orthopedic dentistry
Consists of Gold – 75%, Silver and copper 8% each, and Platinum – 9%
Platinum gives this alloy elasticity and reduces shrinkage during casting.
Used for the manufacture of cast gold parts of clasp dentures, clasps and inlays
- Dental gold alloy 750 standard (ZlSrKdM)
Cadmium is added to the composition - 5-12%.
Due to cadmium, the melting point of the alloy is reduced to 800 C. (The average melting point of gold alloys is 950-1050 C.) Which allows this alloy to be used as solder.
Silver-palladium alloy in dentistry
Silver-palladium alloys are distinguished by a higher melting point = 1100-1200 C. Their physical and mechanical properties are similar to gold alloys. But corrosion resistance is lower. (Silver darkens when in contact with sulfur compounds) Alloys are ductile and malleable. Soldered with gold solder (ZlSrKdM).
COMPOSITION: 75.1% silver, 24.5% palladium, some alloying metals (zinc, copper, gold).
Used for stamped crowns. They are produced respectively in the form of disks of various diameters (18, 20, 23, 25 mm) and 0.3 mm thick.
Composition: 78% silver, 18.5% palladium, other metals.
Used as an alloy for casting in dentistry.
The amount of palladium was reduced to 14.5%, silver was increased.
Classification
Metals are classified into two main groups - ferrous and non-ferrous. Ferrous materials include iron, iron-carbon alloys, cast iron, and steel.
Non-ferrous - aluminum, copper, zinc, nickel. Modern dentists use more than 400 alloys based on various metals.
They are divided into the following groups:
- Noble.
- Ignoble.
- Solders (precious or non-precious materials used for soldering).
Noble
The cost of noble metals and the alloys they contain is quite high. At the same time, they enjoy deserved popularity because they have an invaluable quality for medical use - biocompatibility.
This is interesting: Metal-ceramics or ceramics: what is the difference, what is better to choose for installing crowns
They are not familiar with the negative effects of corrosion; they do not come into contact with salivary secretions, which are constantly present in the oral cavity and have a detrimental effect on metal structures of a more “primitive” content.
Components made from precious elements do not cause individual intolerance to the material, fraught with outbreaks of allergies, and do not belong to the group of compounds that can cause intoxication as they accumulate in the body.
Due to the described factors, gold or platinum alloys are often the only possible solution for patients prone to polyetiological contact intolerance.
The main advantage of noble metals is their almost lifelong service life. Well, the disadvantages include, perhaps, their too high cost, in comparison with analogue offers made from cheaper components.
The main area of application of noble metals is bridges and stamped crowns. Platinum is an ideal solution for the manufacture of cast-type clasp prosthetic fragments, inlays and clasp retainers.
Properties of noble alloys
The ideal option, according to most dentists, is expensive gold-platinum alloys.
They have impeccable mechanical and physical properties, high biocompatibility and are well combined with ceramics. The most famous noble alloys produced in Russia are “Plagodent” and “Plagodent Plus”. Palladium in combination with gold forms alloys with excellent physical and mechanical properties and high corrosion resistance. They have a decent yield strength and high tensile strength, so long bridges and permanent elements of locking joints are made from them. In appearance they resemble base alloys. Only 10% palladium in the composition gives the alloy a white color, similar to steel.
Working with palladium structures requires certain skills from the dentist due to the high melting point of the alloy and special casting techniques. Soldering and laser welding should be avoided. Gold-palladium alloys are cheaper than gold-platinum alloys: due to different densities, the difference in cost can reach 2-2.5 times.
The percentage of noble metal in the alloy affects corrosion resistance and biocompatibility. The cost of a prosthesis can only be reduced by switching to non-precious alloys. A noble alloy may contain a high or low gold content, or may not contain this element at all, as, for example, in silver-palladium compositions.
What determines the color of noble alloys?
Doctors pay attention to color. It is believed that a very yellow alloy containing a lot of gold improves the color of ceramics: the oxides of such alloys are easily coated with a thin layer of opaque and look aesthetically pleasing. A patient who observes the golden color of the crown frame at intermediate stages has no doubt about the need to purchase such an expensive prosthesis.
In some noble alloys, the proportion of palladium, platinum or silver is increased, so they lose their yellow color, but remain just as strong. Such varieties are suitable for the manufacture of extended bridges, with or without implant support. If you are going to install a single crown, you can get by with an option with a high gold content and a low content of platinum group metals.
Alloys with fewer noble components have a pronounced yellow color and do not have such good properties as white alloys. They contain a high content of indium, which in combination with palladium gives a bright straw color. They do not have sufficient elasticity and corrosion resistance. They are used mainly in China and India on a massive scale to reduce the cost of dentures.
Ignoble
Base metals have a single purpose in their use - reducing the cost of prosthetic structures. They are based on environmentally friendly components that can replace gold or platinum.
Stainless steel, chrome-cobalt combinations of metals, and nickel-chromium compounds are best suited for this.
Such components are the most popular metals in orthodontic practice. In addition, they have excellent alloying characteristics, so that the composition can be given any properties.
This is interesting: Zirconium dental crowns made of zirconium dioxide for the front and chewing teeth: before and after photos
The main advantage of stainless steel is its low cost. Disadvantages can be considered:
- heavy specific gravity - density about 8 grams per cubic cm;
- high chemical activity;
- the ability to provoke allergic reactions;
- tendency towards galvanosis.
Application area:
- fixed type of prosthetics;
- personal crown frames;
- cast dental systems;
- facets;
- sleeves of a standard type, which are a template blank for the manufacture of stamped crown products;
- clasps for fixing dentures and bracket systems;
- filling matrices;
- separation plates;
- soldered bridges;
- orthodontic wire.
Base compounds have good mechanical and chemical properties:
- low density;
- fluidity is within normal limits;
- meltability.
Dental stainless steel alloy - dental steel
Steel is the most common alloy in the world. Its properties are well known. And due to alloying agents, it can be given any desired properties.
Dental steel is very cheap.
Among the disadvantages: steel is heavy (density about 8 g/cm3) and chemically active. May cause allergies, galvanosis.
Stainless steel in orthopedic dentistry - grades:
- STEEL GRADE 1 X 18 H 9T (EYA-1)
Dental alloy for crowns COMPOSITION:
1.1% carbon; 9% nickel;18% chromium; 2% manganese, 0.35% titanium, 1.0% silicon, the rest is iron.
Used for fixed prostheses: individual crowns, cast teeth, facets.
- STEEL GRADE 20Х18Н9Т
COMPOSITION: 0.20% carbon, 9% nickel, 18% chromium, 2.0% manganese, 1.0% titanium, 1.0% silicon, the rest is iron.
The following are produced from this type of steel in the factory:
- standard sleeves used for the production of stamped crowns;
- elastic metal matrices for filling, as well as separation strips
STEEL for dentistry GRADE 25Х18Н102С
COMPOSITION: 0.25% carbon, 10.0% nickel, 18.0% chromium, 2.0% manganese, 1.8% silicon, the rest is iron.
APPLICATION: manufactured in the factory:
- teeth (lateral upper and lower) for stamped-soldered bridges;
- frames for metal-plastic bridges, for cladding;
- orthodontic wire with a diameter of 0.6 to 2.0 mm (step 0.2 mm).
Silver solder PSR-37 or Cet solder is used as solder for non-precious alloys
Rina.
Contains silver - 37%, copper - 50%, Manganese - 8-9%, Zinc - 5-6%
Melting point – 725-810 C
Why, anything else?
The negative impact of metal inclusions in the oral cavity has been studied since the end of the 19th century. All metals used in orthopedic dentistry are destroyed under the influence of mechanical factors and the chemical composition of saliva. The most severe pathological changes were observed in patients with prostheses made of dissimilar metals and it was recommended to especially avoid the gold-stainless steel pair. Metal fillings and crowns change the ionic composition of saliva: a direct relationship has been established between trace elements and microcurrents in the oral cavity. Gallodent, amalgam and stainless steel are especially distinguished in terms of the height of electrical potentials. Electrogalvanic and allergic complications are based on corrosion, and the aggravating factor is the period of use of the prosthesis. As a result of the interaction between the tissues of the oral cavity and saliva, Co, Cr, Ni penetrate through the mucosa and have the ability to accumulate. Metal inclusions, especially in combination, have an adverse effect on the receptor apparatus of the oral cavity and the body as a whole. When using stainless steel prostheses, a large amount of Cr is released, which accumulates in the internal organs. The toxic effect of chromium is manifested by dystrophic changes in the kidneys, the appearance of protein in the urine, dysfunction of the gastrointestinal tract, gastric and duodenal ulcers. KHS is not without its shortcomings. It can also cause pathological changes in the oral cavity. Cobalt and nickel are allergens (haptens) and cause allergic lesions of the oral mucosa in people who have dentures made of stainless steel and chromium-cobalt alloy. The most active allergen in terms of the frequency of reactions caused is nickel. When using these dentures, patients experience a feeling of acidity, burning in the mouth, dryness, and discomfort. Most often this occurs as a consequence of the manifestation of electrochemical corrosion and the occurrence of microelectrogalvanic currents. To eliminate the unfavorable background, all inclusions must be replaced with indifferent ones, rather than avoiding dissimilar metals. The negative impact of CCS on human organs has led to the need to search for new materials with improved characteristics for orthopedic treatment of patients. The use of titanium alloys for otropedic dentistry has recently attracted great interest.
Cobalt chromium alloy in dentistry
(cobalt-chromium alloy, cobalt-chromium alloy)
COMPOUND:
- cobalt 66-67%, alloy base, hard, durable and light metal.
- chromium 26-30%, introduced mainly (as in steel) to increase corrosion resistance.
- nickel 3-5%, increases ductility, malleability, toughness of the alloy, improves the technological properties of the alloy.
- molybdenum 4-5.5%, increases the strength of the alloy.
- manganese 0.5%, which increases strength and casting quality, lowers the melting point, and helps remove toxic sulfur compounds from the alloy.
- carbon 0.2%, reduces the melting point and improves the fluidity of the alloy.
- silicon 0.5%, improves the quality of castings, increases the fluidity of the alloy.
- iron 0.5%, increases fluidity, improves casting quality.
PROPERTIES of KHS dental alloy:
It is distinguished by good physical and mechanical properties, low density (and, accordingly, the weight of restorations) and excellent fluidity, allowing the casting of openwork products of high strength.
Melting point is 1458 C
The alloy is resistant to abrasion and retains a mirror-like shine for a long time.
Cobalt-chrome alloy in dentistry
Used for cast crowns, bridges, solid clasp dentures, frames of metal-ceramic dentures, removable dentures with cast bases, splinting devices, cast clasps.
Metal-ceramics metal composition in dentistry
metal alloy
metal ceramics in dentistry.
Stump inlay made of cobalt-chrome in Moscow
In a dental clinic in Moscow, a crown can be installed on a tooth either on a pin or on a special inlay for the crown, called a pin stump inlay. They are needed to restore especially thin or almost destroyed tooth walls. After restoring the tooth using a pin stump, a crown is placed on it, which is secured with a special cement mortar. A post stump inlay is a dental product that is made on the basis of an individual impression and has 2 parts: root and coronal. The first is fixed in the canal of a semi-resolved tooth, the second - under the crown instead of the dental stump.
Pin stump inlays are made individually for each tooth, taking into account its structure and the size of the roots. The outer material for the post stump inlay is cobalt-chromium alloy (CHS), gold, ceramics, etc. To obtain the required result, it is necessary that the material from which the pin stump inlay for the crown will be made meets the requirements listed below:
- must be bioinert;
- guarantee high-quality adhesion to the casting.
When listing the advantages of cobalt-chrome inlays, it is worth highlighting their reliability, long service life and ease of use. Due to the fact that this tab is placed in each memoral canal - multi-channel stump tabs, it guarantees a uniform load, and the use of a special cement composition promotes excellent fastening and tightness, eliminating the possibility of gaps appearing where food could remain. Teeth with core inlays do not have a warranty period. A chrome-cobalt core inlay is a one-piece or dismountable structure made of a cobalt-chrome alloy, which is placed on the tooth root and serves as the basis for the crown. Before fixing the cobalt-chrome crown, endodontic treatment is carried out, when the dentist meticulously fills the tooth canals and forms a prosthetic bed. After which the KHS tab is fixed with special dental cement, and then a permanent crown is installed. Chromium-cobalt is an alloy widely used in dentistry to create dentures and implants. This alloy does not oxidize under the influence of food and saliva, retains color and is biocompatible. From cobalt-chrome alloy, it is possible to recreate the most identical dental impressions.
The main stages of preparing and fixing a cobalt-chrome tab
Prosthetics with intraradicular inlays made from CHS has certain limitations.
- Restoring a tooth with a core insert is possible when the tooth is healthy and free of inflammation.
- At the 1st stage, a detailed x-ray examination of the tooth root and gums is carried out. If periostitis or a cyst is detected, surgical treatment is performed. Parts of the tooth affected by caries are also eliminated.
- Stage 2 involves filling the canals and creating a cobalt-chromium inlay.
- At the next stage, the cobalt-chrome pin insert is tried on and secured. The most effective composition for attaching the tab is composite cement.
- At the final stage, a dental crown is made and fixed.
You can find out the price of a stump pin in our Clearstome dental clinic in the Prices section or by calling for a free consultation by phone or 8(968)444-14-14. Call and make an appointment!
Nickel-chromium alloys in dentistry
Alloys in which the main element is Ni. The elements of this alloy, in addition to nickel, are Cr (at least 20%), Co and molybdenum (Mo) (4%).
The properties of the nickel alloy are close to the cobalt alloy.
Used: for casting fixed dentures and removable denture frames.
Today, the use of nickel alloys is limited due to their high allergenicity.
Titanium alloys in orthopedic dentistry
In dentistry, both pure titanium (99.5%) and its alloys are used.
Purpose
Several decades ago, dentists made medical structures exclusively from noble metals in their pure form. Gradually, a trend emerged to use them as various alloys.
Closer to the 20th century, orthopedic dental appliances made of base metals , in particular alloys of steel and chromium, were common.
Base metals are very hard, which complicates their processing, but thanks to this feature, prostheses can be made of any length.
Pure gold was no longer used due to its insufficient hardness. Today, gold-palladium, gold-platinum, silver-palladium alloys are in demand because they are more durable, resistant to corrosion and have many other positive characteristics.
Base metal alloys are also popular.
They are excellent for ceramic coatings because the oxide film that appears on the surface promotes a strong bond.
Auxiliary alloys used in orthopedic dentistry
Bronze is an alloy of copper and tin. Aluminum bronze (aluminum instead of tin) is used in dentistry. It is used to make ligatures for splinting jaw fractures.
Brass is an alloy of copper and zinc; pins for collapsible models are made from it.
Magnalium is an alloy of aluminum and magnesium; aircraft parts are made from it (the alloy is very light and durable). In dentistry, articulators and some cuvettes are made from it.
Amalgams are an alloy of metal and mercury. Used for filling.
The topic is too broad; amalgam in dentistry will be discussed in a separate article.
Low-melting alloys in orthopedic dentistry
Low-melting alloys (Mellota, Wood, Rose) - contain Bismuth, Tin, Lead
– their melting point is about 70 C.
They are used for dies when stamping crowns, counter dies, and making collapsible models.
Low-melting metals in dentistry
Wood's alloy.
Melting point 68 C.
Composition: Bismuth – 50%, Lead – 25%, Tin – 12.5%, Cadmium – 12.5%.
Toxic because it contains cadmium.
Mellot alloy.
Melting point 63 C
Composition: Bismuth – 50%, Lead – 20%, Tin – 30%.
Rose alloy for dentistry.
Melting point 94 C.
Composition: Bismuth – 50%, Lead and Tin 25% each.
Steel for dental instruments
Tool steel – contains carbon from 0.7% or more.
It is characterized by high strength and hardness (after special temperature treatment).
The addition of tungsten, molybdenum, vanadium and chromium to steel makes the steel capable of cutting well at high speeds. This steel is used for burs and cutters.
Tungsten carbide is not an alloy. A chemical compound of tungsten with carbon (chemical formula WC). Comparable in hardness to diamond. Used for the production of armor-piercing tank shells. And also for carbide dental burs.
Zirconium metal in dentistry
Zirconium dioxide is also not an alloy. Chemical compound of zirconium metal with oxygen. Its chemical nature is similar to ceramics, but it is harder and more durable. In dentistry they are used for the manufacture of milled dentures.