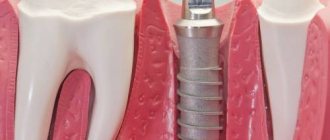
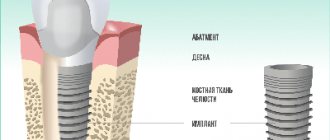
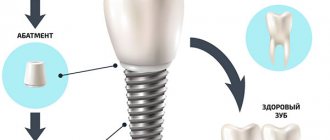
Osseointegration in dental implantation is the process of engraftment of a dental implant into bone tissue. If the effect is positive, the jaw bone “accepts” the metal artificial root, does not reject the foreign body, and subsequently functions normally.
The very concept of “osseointegration of an implant” became popular at the end of the 20th century, when implantology began to actively develop in modern dentistry. This process was first discussed in 1965, when Swedish scientists studied the process of bone tissue regeneration after human injuries.
Factors influencing the success of osseointegration
The success of implantation of an artificial root depends on:
- initial diagnosis;
- type and quality of the implant;
- surgical technique during surgery;
- surfaces and shapes of artificial roots;
- individual characteristics of the body;
- presence of allergies;
- patient's age;
- general condition of the oral cavity;
- past diseases, etc.
Important! The body’s successful “response” to the introduction of an artificial root is not a 100% guarantee of the success of the entire implantation process, although it is objectively considered a key point. In some cases, even with successful osseointegration, the location for installing the implant may be poorly chosen, or a low-quality prosthesis may have been selected.
The material and shape of the implant play a role
For successful engraftment into the bone, the artificial root material must be inert so as not to cause a negative reaction from the body. The following materials meet this requirement:
- bioinert – titanium alloy, zirconium, titanium nickelide, gold, etc.
- biotolerant - an alloy of chromium and cobalt;
- bioactive - metal coated with a calcium phosphate or ceramic layer, porous coating.
Based on the experience of introducing artificial roots, and the general statistics of implant engraftment, the following are considered the best:
- screw
- cylindrical;
- in the shape of a natural tooth;
- with cortical overlays, etc.
Main stages of osseointegration
- Before implantation, the patient’s bone tissue is examined: structure, density, volume, structure, etc. The level of tissue resorption, the ratio of spongy substance and the so-called cortical plate are determined;
- If the bone volume is not enough, augmentation or sinus lift is performed. All this is done to avoid mechanical overload, which can negatively affect the process of root engraftment.
- After insertion, the implant should not be loaded to avoid the formation of a fibrous capsule.
- After 3 to 6 months, the trabecular bone begins to strengthen. In general, it takes about one and a half years for complete and final strengthening of the bone. But, if after six months the process is successfully completed, the new root can be safely loaded with a crown.
- 5. During this period, it is important to be observed by the treating dentist in order to monitor the process visually and using special equipment. If necessary, the specialist will prescribe a course of treatment to speed up and improve the quality of bone integration.
Note! The first two to three weeks after root penetration are indicative for its engraftment. It is in the first days that the so-called trabecular bone is formed around the pin. That is why a crown is not placed on the root, so as not to disrupt the primary process of osseointegration.
The good news is that modern technologies provide high guarantees of successful implantation. In order for the root to take root quickly, it is important:
- install an implant from a trusted manufacturer;
- seek help from an experienced implant dentist who specializes in installing artificial roots;
- follow the recommendations after implantation of the root (regularly brush your teeth, follow a diet, do not load the operated area, stop drinking alcohol and smoking).
Period of complete engraftment
The healing time of dental structures on the upper and lower jaws is different. For the lower one, 4 months is enough. The upper one needs a longer period. On average, it takes about six months . The difference in timing is due to different tissue densities and the resulting load. The upper jaw has less density and the load on it is not so noticeable.
After the first stage of engraftment, an orthopedic structure is made. This is necessary to obtain chewing pressure on the implant. The resulting load will act on the bone, stimulate cell growth, and prevent recession. Complete osseointegration will occur one and a half years after installation.
Achieving contact between bone and dental structure does not guarantee the success of implantation. Sometimes rejection can occur due to improper placement of the implant or poor-quality prosthetics.
Complications of unsuccessful osseointegration
The most common complication is reimplantitis, that is, inflammation around the implanted artificial root. With this diagnosis, the bone tissue gradually decreases, and eventually the root is rejected. The problem arises due to an accidental infection in the socket, improper oral care after implantation, heavy load or trauma to the bone, excessive size of the bone bed and other factors.
To correct the condition, the implant is removed from the socket, the abscess and gum pocket are removed. The gums are treated with an antibacterial composition, and physiotherapy is prescribed to restore soft tissues. After a few months, re-implantation is scheduled.
A little history
The phenomenon of osseointegration was discovered by the Swedish scientist Per-Ingvar Brånemark, conducting research at the University of Gothenburg. In 1965, he studied the processes of restoration of bone structures after injury. Experiments were carried out on rabbits. A titanium sensor was installed in the animal's hip bone. As time passed, it was discovered that it was impossible to remove the sensor - it had fused with the bone tissue. Thus, it was found that titanium is bioinert , that is, compatible with human tissue. The discovery of the phenomenon was very significant for many areas of medicine. In dentistry, the discovery marked the beginning of the development of dental restoration systems.
How to choose a clinic in Moscow to guarantee successful osseointegration?
The 32 Dent clinic chain specializes in dental implantation. That is why, according to statistics, 32 Dent patients have almost 100% successful osseointegration. High-quality implants made in Israel, Switzerland, the USA, the work of experienced specialists, modern equipment - all this is the key to the successful implantation of a new tooth. You should make sure of this by making an appointment with an implantologist now!
If you have a problem similar to that described in this article, be sure to contact our specialists. Don't diagnose yourself!
Why you should call us now:
- We will answer all your questions in 3 minutes
- Free consultation
- The average work experience of doctors is 12 years
- Convenient location of clinics
Single contact phone number: +7
Make an appointment
Sources:
- Personal experience as an implant dentist;
- Vorobyov, A. A. A look at the problem of dental implantation in the light of modern scientific ideas / A. A. Vorobyov, V. I. Shemonaev, D. V. Mikhalchenko, A. S. Velichko // Volgograd Medical Scientific Journal. - 2009;
- Sirak, S. V. Direct dental implantation in patients with included dentition defects / S. V. Sirak, A. A. Sletov, K. S. Gandylyan, M. V. Dagueva // Medical Bulletin of the North Caucasus. - 2011;
- Esposito, M. Interventions for replacing missing teeth: different types of dental implants / M. Esposito, Y. Ardebili, H.V. Worthington // Cochrane Database Syst. Rev. - 2014;
- Dental implants are a viable alternative for compensating oligodontia in adolescents / S. Heuberer, G. Dvorak, C. Mayer, G. Watzek, W. Zechner // Clin. Oral Implants Res. — 2015;
- Immediate loading implants: review of the critical aspects / L. Tettamanti, C. Andrisani, MA Bassi, R. Vinci, J. Silvestre-Rangil, A. Tagliabue // Oral Implantol. — 2017;
- Paraskevich, V. L. Dental implantology: basic theory and practice / V. L. Paraskevich. - Minsk: Unipress, 2002;
- Labis, V.V. The role of the bacterial factor and the immune system in the process of reparative osteogenesis during dental implantation / V.V. Labis, E.A. Bazikyan, I.G. Kozlov // Bulletin of the Orenburg Scientific Center of the Ural Branch of the Russian Academy of Sciences. - 2013;
- Aleinikova, E. V. Modern concept of osseointegration of dental implants / E. V. Aleinikova, A. B. Shabanovich // Medical journal. - 2006;
- The influence of physicochemical properties of the surface of titanium implants and methods of their modification on osseointegration indicators / G. A. Volozhin, A. P. Alekhin, A. M. Markeev, D. V. Tetyukhin, E. N. Kozlov, M. A. Stepanova // Institute of Dentistry. — 2009.
According to WHO, 75% of the population in various regions of the world are partially edentulous, and approximately 30% of people aged 65–74 years are completely missing natural teeth [1]. Against the backdrop of such a high prevalence of edentia, the market for dental implants is developing [2–7]. The estimated US and European dental implant market is expected to reach US$4.2 billion by 2022 [6].
Compared to traditional removable orthopedic structures, dental implants have better functionality, significantly improve the patient’s quality of life and provide a better aesthetic effect [8–10].
One of the main conditions for successful dental implantation is sufficient primary stabilization of the implant in bone tissue, on which the survival of the implant as a whole directly depends. Microexcursion of more than 50–100 μm causes the formation of fibrous connective tissue and bone resorption at the interface between the bone and the implant, which negatively affects osseointegration and bone remodeling [11–13]. The primary stability of implants has become even more important due to the introduction of early and immediate loading protocols into practice [14, 15].
Primary stability depends on the geometry of the implant, its topography, and osteotomy protocols, which regulate the stress applied to the bone tissue in the immediate vicinity of the implant [16].
In the case of an optimal course of the integration process, direct contact is formed between the dental implant and adjacent bone structures without the participation of an intermediate connective tissue layer. This type of connection between the implant and bone tissue is called osseointegration [5, 17, 18].
The main factor determining the stability of the implant is the characteristics of its surface [17, 11, 19, 20]. All the most important research carried out in recent years in the field of increasing the efficiency of dental implants is, as a rule, associated with the development of surface treatment technologies [14, 21—23].
At the first stages of integration, the macrotopography of its surface plays an important role in ensuring the primary stability of the implant. In addition to the shape of the intraosseous part (cylindrical/conical), this includes the degree to which the implant matches the size of the channel drilled in the bone tissue and the presence of large cuts on the implant [24, 25].
Optimization of the osseointegration process and improvement of the mechanical properties of the surrounding bone tissue are also facilitated by the microtopography features of the implant. The presence of microdefects on the implant surface increases the contact area and allows osteogenic cells to attach to the implant surface with the help of structural proteins and glycoproteins, thereby promoting osteogenesis [26, 27].
The surface structure, morphology and chemical composition of a dental implant can be changed in two ways: chemical and physical [28]. The most commonly used method to modify the surface of an implant at the nanoscale is chemical. It includes anodic oxidation, acid etching, alkaline etching, a combination of anodic oxidation and etching, hydrogen peroxide and sol-gel processing, as well as chemical deposition [29–32]. Acid etching of the implant surface (hydrochloric, sulfuric, nitrous and hydrofluoric acids are used) leads to the formation of micro-grooves with a diameter of 0.5 to 2 microns on the surface of the implant. This creates a uniform roughness, increased active surface area and improves cell adhesion, and therefore ensures optimal osseointegration [30, 32, 33]. One of the methods used to treat the surface of implants is to increase the thickness and change the crystal structure of the titanium oxide layer on the surface by anodizing. The peculiarity of electrochemical anodic oxidation is the possibility of creating an effective implant neck surface in contact with soft tissues, resulting in the formation of a gingival papilla [29, 31, 34].
Among the physical methods of modifying the surface of an implant, sandblasting is the most commonly used [35, 36]. Sand particles (silicon oxides, aluminum oxides and/or titanium oxide), falling on the surface of the implant, form microindentations on it. Roughness depends on the size and mass of particles, as well as the speed of their movement, exposure time, and the distance from the spray source to the implant surface [37, 38]. Since after processing some particles are firmly imprinted into the surface, acid etching with hydrofluoric, nitric or sulfuric acids is often carried out to homogenize the surface microprofile [39]. When cells come into contact with the developed surface of the implant, their adhesion, proliferation and differentiation of osteoblasts improve.
Recently, several new coating technologies have been developed by applying hydroxyapatite and calcium phosphates (CaP) to the surface of implants [40–42]. It has been proven that CaP promotes the formation of a direct connection with bone tissue compared to implants without additional titanium surface coating [41]. Clinical studies of implants of this type are limited, but there is reason to believe that they may be more effective with early functional loading [43, 44].
In addition, one of the most common surface treatment methods is laser ablation, which makes it possible to create microcavities—cavities several micrometers in diameter with an ordered structure [45, 46].
A separate group consists of bioactive modifications of the surface of dental implants [47, 48]. Various osteoinductive growth factors are mainly used [49–51]. In addition, to reduce the risk of developing infectious complications, the application of antibacterial drugs to the surface of implants is widely used [3, 52, 53].
Another important factor influencing osseointegration is hydrophilicity/hydrophobicity. In a study by R. Baier, D. Meyer (1988), it was shown that hydrophilic materials (surface tension above 30 dynes/cm) interact more closely with biological fluids, cells and tissues and, accordingly, contribute to a better process of osseointegration [18, 54] . The roughness of the implant surface also plays a certain role in this process. Fibroblasts and epithelial cells adhere more strongly to smooth surfaces, and the ability for osteoblast proliferation and collagen synthesis is more pronounced on surfaces with moderate roughness [55, 56].
The general principle for modern implant dental structures is to achieve an optimal biomechanical state through strict adherence to the surgical installation protocol, ensuring regeneration and remodeling of bone tissue around the implant [57, 58]. In 95% of cases, this goal is achieved through a successful osseointegration process. In this regard, ensuring optimal conditions for the osseointegration process is the key task of any dental surgeon [59, 60]. In addition to the characteristics of the implant surface, other factors that influence osseointegration include implant design, characteristics of the implant material, the condition of the adjacent bone, surgical technique and implant loading [59, 61–65].
The main requirement for the material selected for implantation is its biocompatibility, which is determined by the chemical composition, mechanical and electrical properties of the material, as well as surface features [67–68]. In addition, the material should not cause toxic reactions, be carcinogenic, allergenic or radioactive [69].
Most of the requirements for materials used in dental implantology meet titanium and its alloys [61, 70—72]. They are characterized by high elasticity and low density, due to which their strength is higher than the strength of other metals. When titanium implants are introduced into tissue, direct contact between titanium and body tissues does not occur, since titanium is capable of forming a thin oxide film on the surface of the implant, which increases the anti-corrosion properties of the material and, thanks to stable and high-density oxides, has high viscosity and good adhesion [73-75] .
A number of other materials are also known that are capable of forming a strong connection with bone. These include zirconium and some ceramic materials [66, 76–80].
There is no consensus among researchers regarding the best shape for implants, but it is generally accepted that the geometric shape should maximize the area of contact between the bone implant and the tissue and reduce stress near the implant neck area [81]. Currently, cylindrical and conical implants are most actively used [82]. The latter have proven themselves to be effective in conditions of low bone density [82–84], since the largest part of the load is distributed in the axial direction, in which the area of the conical implant is larger [85, 86].
The stability of implants is significantly influenced by the shape of the connection with the prosthesis [87]. Screw implants dominate the global dental market. The screw surface provides a larger contact area between the implant and the bone, increases primary stability, reduces shear stress at the bone implant interface and stress concentration in the implant neck area [88, 89]. For multi-tooth implants, more complex connection forms are used - hexagonal connections and Morse taper connections [13, 90, 91].
Among the surgical factors that influence osseointegration, preparation of the bone bed is critical [92–95]. Mechanical and thermal tissue damage that occurs during the formation of the implant bed can have a destructive effect on the original bone tissue and the result of surgical treatment [96, 97]. If the drilling temperature during the formation of the bone bed exceeds 47 °C for 1 min, then thermal osteonecrosis may occur - a fibrous capsule is formed around the implant, and the strength of its connection with the bone is significantly reduced [17]. In an experimental study by S. Yeniyol et al. [98] found that the optimal bed drilling speed from the point of view of the success of the osseointegration process is 1000 rpm [98]. The technique for preparing the bone bed for the implant is also of great importance. For example, S. Stacchi et al. [99] in a randomized controlled clinical trial showed that the stabilization rate of implants installed using piezoelectric instruments decreased by 2.7% on the 21st day, and when installed using rotary cutters - by 9.2% ( p
<0,0001) [99].
Overall implant stabilization can be considered a combination of primary and secondary stabilization [100]. As noted earlier, primary stability, determined by mechanical interlocking between bone and implant, without biological interaction, plays a key role in successful osseointegration. Primary stability depends on the geometry and topography of the implant, as well as osteotomy protocols, which regulate the stress applied to the bone tissue in the immediate vicinity of the implant [16, 17, 101].
Thus, the result of dental implantation is determined by the multifactorial nature of the process, and its success depends on the consolidated efforts of implant manufacturers and the specialist performing this procedure.
The success of dental implant treatment largely depends on the condition of the jaw bone tissue [102–104]. According to a number of authors, the incidence of complications in patients with osteopenia and osteoporosis in the early and late postoperative period can vary from 5 to 56% [105, 106]. Therefore, even at the very initial stage of implantation, it is necessary to clearly determine the volume of bone tissue and determine the quality of the bone of the alveolar part of the jaw [107, 108].
The simplest clinical way to assess implant stability is through direct tactile perception by the surgeon. However, it is clear that such experience cannot form the basis of effective implantation protocols [15, 109].
The method of primary stability prediction, based on a computer calculation of the energy spent on drilling a certain volume of bone tissue, allows you to estimate the density of bone tissue in the implantation area, which correlates with its primary stability [19, 109].
A number of methods, combined as modal analysis, are based on processing the response of the implant to a certain external physical influence. These include the percussion test, the impact method, the pulsating oscillation method, etc. [109-111]. A more objective and common method for measuring implant stability is based on standard physical stimulation and analysis of the audio response using fast Fourier transform or other signal processing techniques [111]. The Dental mobility checker method also operates on a similar principle [40].
Two-dimensional radiography allows one to assess the density of bone tissue in the implantation area and identify areas of low density, thus predicting the further stability of the implant [111]. The most accurate radiological method is cone beam computed tomography [112, 113].
Resonance frequency analysis is widely used in dental practice in studies devoted to the use of various implantation modes, types of implants and the study of factors affecting osseointegration [114, 115]. Modern devices for resonance frequency analysis, unlike other devices for assessing implant stability, are non-contact and do not affect the condition of the implant [116, 117].
Currently, from the point of view of functional load in dental implantology, there are two methods for solving this issue: the traditional method of delayed functional load and the method of early load, which has been actively developing in the last three decades [12, 70, 81, 118]. The latter is widespread due to the introduction of a one-stage implantation protocol into clinical practice [119–123].
Early loading indicates the possibility of applying occlusal loading to dental implants before the traditional healing period of 3 to 6 months has passed [124–126].
The effectiveness of the early loading protocol alone and in comparison with the traditional delayed loading technique has been studied in many experimental and clinical studies [70, 119, 127–130].
R. Mura (2012) [131] studied the effectiveness of an early loading protocol in 48 patients (79 implants). The five-year implant survival rate was 100% and the average bone loss rate was 0.56 mm. L. Bogaerde et al. (2010) [132] in their study ( n
=21, 69 implants) note the survival rate of implants subjected to early functional loading at 98.5% during the observation period (18 months). In this case, the average marginal bone loss was 0.7 mm, and the average implant stability coefficient was 73.7. The authors note that the effectiveness of treatment is comparable to the results of the protocol with delayed loading.
A. Pozzi et al. (2015) [133] studied the effectiveness of immediate loading during direct installation of implants for 3 years ( n
=54, total 118 implants).
According to the study, only 1.9% of implants were lost due to peri-implantitis in smoking patients. In a study by A. Pozzi (2016) [134], 148 implants (NobelReplace) were placed both in post-extraction sockets ( n
= 67) and in already healed sockets (
n
= 81).
The cumulative success rate was 99.3%. The average bone loss 2 years after implantation in post-extraction sites was 0.69 ± 0.75 mm, and in healed sockets 0.62 ± 0.80 mm (the difference did not reach statistical significance) [134]. Another study did not reveal significant differences in the stability of BoneTrust plus dental implants installed in the socket of an extracted tooth, with immediate loading with temporary fixed prostheses and with delayed loading ( p
> 0.5) [37].
Zh.A. Ashuev (2012) [70] in an experimental study on laboratory mini-pigs showed that with early functional load on dental implants, hyperemia develops in regional vessels and the microvasculature of supporting tissues, which is accompanied by increased tissue blood flow and vasomotor activity of microvessels, thereby ensuring the process osseointegration. The functional activity of the masticatory muscles increases during early functional loads on dental implants, and their coordinated work is restored after 3 months [70].
At present, the social significance of edentia cannot be underestimated. Functional and aesthetic problems associated with tooth loss sharply reduce the quality of life of patients and cause various types of psychological disorders [122, 135-137]. The functional load regime directly affects, on the one hand, the condition of bone tissue, and, on the other hand, the patient’s quality of life in the period after implantation [100].
A.A. Kulakov and Zh.A. Ashuev (2007) [138], based on data from their experimental studies, note that immediate functional load has significant advantages over the traditional delayed load protocol. First of all, this is a reduction in the number of traumatic stages of intervention and postoperative restrictions, a significant reduction in treatment time, maintaining the height and width of the alveolar bone at a constant level, as well as accelerating the processes of bone regeneration [138]. A clinical study conducted by G. Romanos (2015) [139] showed that by functionally loading the bone immediately after immediate implantation, bone resorption can be controlled [139]. A significant advantage of the early loading method is the possibility of restoring teeth even with advanced periodontal disease [140, 141].
Thus, the main problem of using an early functional loading protocol is to ensure primary stability of the implant [142], and the main reasons for unsuccessful implantation are low density and quality of bone tissue [143, 144]. In addition, when planning immediate functional loading, great importance is attached to the choice of implant shape. The literature indicates that the most preferable choice is threaded implants, which have a large contact area with bone tissue and are less mobile [145]. Additionally, stability is increased by the presence of microthreads on the neck of the implant. However, good bone health may compensate for poor implant design [146–147].
Dental implantation is becoming more and more accessible to a wide range of consumers. At the same time, modern methods and means used in dental implantology make it possible to carry out the procedure under almost any anatomical conditions.
Due to the importance of early rehabilitation of patients with partial and complete loss of teeth when using dental implants, the problem of finding the optimal timing of the functional load on dental implants is becoming increasingly urgent. Most published studies are experimental in nature and limited to small observational sizes. Thus, further research in the field of methods for optimizing the contact between the implant and the bone, as well as the mechanisms of osseointegration, depending on the timing of the functional load, will potentially improve the quality of treatment when using implants.
The authors declare no conflict of interest.
The authors declare no conflicts of interest.
Information about authors
For correspondence:
Porfenchuk Dmitry Aleksandrovich - postgraduate student of the 2nd year of study at the Federal State Budgetary Institution "TsNIIS and Maxillofacial Surgery" in the department of clinical experimental implantology; e-mail; tel.: +7(903)244-4292