Sources of influence
Arsenic is a naturally occurring element in the earth's crust and is widely distributed in the environment - in air, water and soil.
Its inorganic form is highly toxic. People are exposed to elevated concentrations of inorganic arsenic through contaminated drinking water, by using contaminated water for cooking and irrigating food crops, during industrial processes, and by eating contaminated foods and smoking tobacco.
Long-term exposure to arsenic in drinking water and food can lead to chronic arsenic poisoning. The most common consequences are skin lesions and skin cancer.
Drinking water and food
The greatest threat to human health is arsenic contained in groundwater. Inorganic arsenic occurs naturally in high concentrations in groundwater in a number of countries, including Argentina, Bangladesh, India, China, Mexico, the United States of America and Chile. Sources of exposure include drinking water, food crops irrigated with contaminated water, and food prepared with contaminated water.
Fish, shellfish, meat, poultry, dairy and grain products can also be dietary sources of arsenic, although the level of exposure to arsenic contained in such foods is generally much lower than the level of exposure to contaminated groundwater. In marine products, arsenic is mainly found in its less toxic organic form.
Industrial processes
Arsenic is used industrially as an alloying additive and in glass, dyes, textiles, paper, metal adhesives, wood preservatives, and ammunition production processes. Arsenic is also used in tanning processes and, to a limited extent, in the production of pesticides, feed additives and pharmaceuticals.
Tobacco
People who smoke tobacco may also be exposed to naturally occurring inorganic arsenic in tobacco because tobacco plants absorb a significant amount of arsenic naturally present in soil. In the past, levels of potential arsenic exposure were much higher because tobacco plants were commonly treated with an insecticide containing lead arsenate.
Arsenic poisoning of the human body
Arsenic poisoning of the human body is divided into acute and chronic. The first option is characterized by large doses of the substance entering the body and symptoms of poisoning appear almost immediately. Chronic intoxication is characterized by long-term absorption of a substance in small doses by the body, but at the same time, its accumulation occurs. Most often this happens when working in chemical plants.
When poisoned, all organs are affected, but especially the nervous and digestive systems. It is worth noting that arsenic not only disrupts the functioning of organs and systems, but also affects cellular development and processes.
Arsenic remains in the body for quite a long time and several weeks after penetration, it can be detected in nails and hair. Bones and teeth are characterized by the same long-term cumulative effect. 90% of the substance can be eliminated through the urinary system, and the remaining 10% through the intestines.
Health implications
Inorganic arsenic has been shown to be a carcinogen and is a major contaminant of drinking water globally. Arsenic also occurs in organic form. Inorganic arsenic compounds (like those found in water) are highly toxic, while organic arsenic compounds (like those found in seafood) are less harmful to health.
Acute consequences
Immediate symptoms of acute arsenic poisoning include vomiting, abdominal pain and diarrhea. They are followed by numbness and tingling in the limbs, muscle cramps and, in the most severe cases, death.
Long term impact
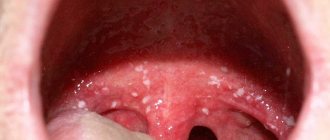
The first symptoms of long-term exposure to high levels of inorganic arsenic (eg, through drinking water or food) usually appear on the skin and include pigmentation changes, skin lesions, and roughening of the skin on the palms and soles (hyperkeratosis). These symptoms appear after exposure for at least five years and may be a warning sign of skin cancer.
In addition to skin cancer, long-term exposure to arsenic can also cause bladder and lung cancers. The International Agency for Research on Cancer (IARC) classifies arsenic and arsenic compounds as human carcinogens and also lists arsenic in drinking water as a human carcinogen.
Other negative health effects that may be associated with long-term inorganic arsenic use include effects on development, diabetes, lung disease, and cardiovascular disease. Arsenic-induced myocardial infarction, in particular, may be a significant cause of excess mortality. In China (Taiwan Province), arsenic exposure has been linked to “black leg disease,” which is a severe blood vessel disease that leads to gangrene. However, this disease is not observed in other parts of the world, so it is possible that malnutrition contributes to the development of this disease. Arsenic is also associated with adverse pregnancy outcomes and infant mortality, as well as health effects in children (1), and exposure in utero and early life is associated with increased mortality among youth due to various types of cancer, lung disease, heart attack, and kidney disease. insufficiency (2). Numerous studies have shown the negative effects of arsenic exposure on mental development, IQ, and memory (3).
"Military arsenic."
After the use of chlorine and other poisonous gases during the First World War, chemists in different countries began to develop even more deadly chemical weapons. They, of course, paid a lot of attention to arsenic. In 1918, the American chemist W. J. Lewis, in search of new components for chemical weapons, reacted acetylene with arsenic chloride in the presence of aluminum chloride. As a result, it formed a dark brown liquid with the smell of geranium, which contained b-chlorovinyldichloroarsine as the main component: AsCl3 + C2H2 ® ClCH=CHAsCl2, as well as b,b'-dichlorodivinyldichlorarsine (ClCH=CH)2AsCl2 and b,b' ,b"-trichlorotrivinylarsine (ClCH=CH)3As. This pleasant-smelling mixture, named lewisite after the chemist, had a terrible blister, generally poisonous and irritating effect. Already at a concentration of 0.3 mg/m3, lewisite vapor causes irritation of the upper respiratory tract, and with increasing concentrations it causes damage to the eyes, skin and death. When droplets of lewisite come into contact with the skin, it is quickly absorbed into it, disrupting the course of many biochemical processes and causing severe damage to the body, especially the vascular system. This circumstance at one time gave rise to the Americans calling lewisite “the dew of death.”
Soon other arsenic poisonous substances were synthesized. Among them was a group of irritating substances, its typical representatives being diphenylchloroarsine (C6H5)2AsCl, diphenylcyanoarsine (C6H5)2AsCN, adamsite:
Substances of this group selectively act on the nerve endings of the mucous membranes - mainly the membranes of the upper respiratory tract. This causes the body to reflexively release the irritant by sneezing or coughing. Unlike tear poisonous substances, these substances, even in mild poisoning, act even after the affected person has escaped from the poisoned atmosphere. Within several hours, a person is shaken by a painful cough, pain appears in the chest and head, and tears begin to flow involuntarily. Vomiting, shortness of breath, and a feeling of fear occur; all this leads to complete exhaustion. And in addition, these substances cause general poisoning of the body.
Fortunately, lewisite and other arsenic toxic substances did not have time to be used in the war, but in all countries, including the USSR, lewisite was accumulated in huge quantities - tens of thousands of tons. It is not easy to neutralize it in a safe way. One of the methods is oxidation to low-toxic arsenic acids:
ClCH=CHAsCl2 + H2O2 ® CHAs(O)(OH)2 + 2HCl;
another way is chlorination with the formation of AsCl3, which is used in industry (see ARSENIC).
Scale of the problem
Arsenic contamination of groundwater is widespread, and a number of areas have significant levels of arsenic contamination in drinking water. Today, at least 140 million people in 50 countries are known to drink water with arsenic concentrations above the WHO guideline level of 10 µg/liter (4).
After in the 1990s. In Bangladesh, widespread arsenic was found in well water, and the issue of arsenic exposure in that country has received a lot of attention. Since then, significant progress has been made, and the number of people exposed to arsenic levels above the Bangladesh Drinking Water Quality Standard has decreased by approximately 40%. Despite these efforts, it is estimated that in 2012, approximately 19 million and 39 million people in Bangladesh were exposed to arsenic levels above the national standard of 50 μg/liter and the WHO guideline of 10 μg/liter, respectively (5 ). In areas of Bangladesh where the problem is particularly acute, 21.4% of all deaths were caused by arsenic concentrations in drinking water greater than 10 µg/liter (6). A similar dose-response relationship was observed in other parts of Bangladesh: these results were combined with those of a national survey, and the combined analysis yielded an annual arsenic-related mortality rate of 43,000 (7). The US National Research Council has noted that lifetime consumption of drinking water containing 50 µg/liter arsenic (8) may be responsible for up to 1 in 100 additional cancer deaths.
Symptoms and signs caused by long-term exposure to elevated concentrations of inorganic arsenic vary between individuals, populations, and geographic areas. Therefore, there is no general definition of the disease caused by arsenic. This makes it difficult to estimate the burden of disease associated with arsenic.
There is also no method for distinguishing between cancers caused by arsenic and cancers caused by other factors. As a result, there is no reliable estimate of the extent of the problem worldwide.
In 2010, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) re-evaluated the health effects of arsenic exposure in the light of new evidence. JECFA concluded that for selected areas of the world where levels of inorganic arsenic in drinking water exceed 50-100 µg/litre, there is some evidence of adverse effects. For other areas where elevated levels of arsenic in water (10-50 µg/litre) are observed, the committee noted that while adverse effects may occur, the prevalence is low and would be difficult to detect in epidemiological studies.
First aid for poisoning
If you suspect arsenic poisoning through the mouth, you should immediately drink a glass of warm water in which three grams of citric acid or one tablespoon of vinegar is diluted.
It is recommended to drink plenty of fluids (the procedure helps not only to quickly remove the poison along with urine, but also fights dehydration of the body caused by vomiting and diarrhea); In no case should you fight vomiting and diarrhea; on the contrary, you need to promote the natural cleansing of the body by drinking warm milk. Taking adsorbents is not a very effective procedure, but in the absence of other means it helps to slightly reduce the dose of poison in the body. If arsenic gets on the skin, it is enough wash the body with warm water and soap, preventing the poison from being absorbed into the blood.
It is important to understand that in case of arsenic poisoning, emergency medical attention is needed. Toxicologists treat arsenic intoxication in the body.
Before treatment, laboratory tests are prescribed. Urine and blood tests can determine the amount of arsenic in the body. It is worth noting that in urine it should not exceed 100 mcg per 1 liter of urine, and in blood 30 mcg per 1 liter. There is also decreased hemoglobin and red blood cells in the blood, increased ESR, and increased protein levels in the urine.
After a few months, arsenic can be easily detected in the victim's hair and nails. There are special drugs that act as an antidote and are administered intramuscularly in a hospital setting; antidote and symptomatic therapy is also performed, and, if necessary, urgent resuscitation measures.
Best materials of the month
- Coronaviruses: SARS-CoV-2 (COVID-19)
- Antibiotics for the prevention and treatment of COVID-19: how effective are they?
- The most common "office" diseases
- Does vodka kill coronavirus?
- How to stay alive on our roads?
Prevention and control
The first action for affected regions is to prevent further exposure to arsenic by ensuring safe water supplies for drinking, cooking, and irrigating food crops. There are a number of ways to reduce arsenic levels in drinking water.
- Replacing sources with high concentrations of arsenic, such as groundwater, with microbiologically safe sources with low concentrations of arsenic, such as rainwater and treated surface water. Water with a low concentration of arsenic can be used for drinking, cooking and irrigation, while water with a high concentration of arsenic can be used for other purposes, such as washing and washing clothes.
- Distinguishing between sources with high and low arsenic concentrations. For example, you can test water for arsenic levels and paint wells or hand pumps with different colors. When combined with effective health education, this can be an effective and inexpensive intervention to quickly reduce arsenic exposure.
- Mixing water with low and high concentrations of arsenic to achieve acceptable arsenic levels.
- Install arsenic removal systems—centralized or individual—and ensure proper disposal of removed arsenic. Technologies for arsenic removal include: oxidation, coagulation-precipitation, absorption, ion exchange and membrane technologies. There are a growing number of effective and low-cost options for eliminating arsenic from small and domestic water supplies, although there is still limited data on the extent to which such systems are used effectively over sustained periods of time.
Long-term interventions are needed to reduce workplace exposure to arsenic during manufacturing processes.
Health education and community involvement are key to ensuring successful interventions. It is important that community members understand the dangers of exposure to high concentrations of arsenic and know the sources of arsenic exposure, including arsenic contamination of food crops (eg, rice) from irrigation water and arsenic contamination of food from cooking water.
Monitoring of high-risk groups should also be conducted to detect early signs of arsenic poisoning, usually skin problems.
Arsenic poisoning in pregnant women
Even small doses of such a substance can harm not only the mother, but also disrupt the development of the fetus. Naturally, if a pregnant woman is poisoned with arsenic, a miscarriage may occur, as well as the birth of a child with various abnormalities. The body of a pregnant woman is weak during this period and even dental treatment with arsenic is prohibited. At this point, the teeth are weakened, which can lead to arsenic falling out and being ingested.
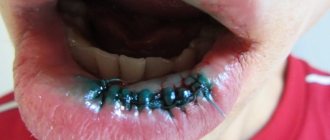
Why does an arsenic filling hurt?
When treating a tooth with arsenic, many hope for an immediate cessation of all torment, but the effectiveness of the product increases gradually. Sometimes it takes 2-3 days for complete necrosis of the nerve. As a result, the doctor should receive a completely devitalized pulp, in which there will be no nerve cells that could hurt. After this, he will have the opportunity to calmly remove the waste soft tissue in order to calmly fill the tooth.
For some time after applying arsenic, the tooth continues to hurt. You can make you feel better with the help of Nurofen, Nimesil, Ketorol, Ketanov.
"Nurofen"
"Ketanov" and "Ketorol"
In this condition it is not recommended:
- They put arsenic, but the tooth still hurts
- take large doses of anesthetics;
- warm up the sore side;
- create mechanical stress on the diseased tooth when chewing.
The use of heating pads and warm compresses is not recommended
Do not get carried away with self-medication and pain relief, so as not to miss the phase of transition from normal devitalization to symptoms of pathology. If a tooth hurts for a long time and constantly, this is a dangerous signal, indicating that serious complications have begun, the most severe of which is sepsis.
Prolonged pain indicates the onset of complications
Why does the tooth continue to ache even after such a strong painkiller as arsenic? You cannot endure such pain for a long time, since the consequences of the “feat” are unpredictable. Prolonged discomfort may be a sign of:
- inaccurately selected dosage (in this case, less than the prescribed norm);
- improper treatment of the cavity with arsenic, in particular – a very dense filling;
- advanced forms of the disease, when arsenic is already powerless: with periodontitis, periodontitis, phlegmon, periostitis, abscess;
Tooth abscess is an infectious process that is caused by the accumulation of pus in the inner or outer surface of the alveolar process of the upper jaw or the alveolar part of the lower jaw
- painful reaction to arsenic and other ingredients of the composition (severe allergies and even anaphylactic shock are possible);
- swelling of the gums, fever and other symptoms of periodontitis;
- necrosis of the periosteum and bone tissue;
Necrosis of hard dental tissues
- swelling of the gums in the area of the problematic chewing organ;
- non-compliance with filling technology with arsenic;
- aggressive effect of the drug on dental tissue;
- spread of inflammatory processes from the pulp to the neighboring area.
The exact cause can only be determined through examination; the main thing is not to put off visiting the dentist.
You can't stand the pain, you need to see a doctor urgently
The most common symptoms that should be a cause for concern are presented in the table.
| Symptoms | Description of the causes of the complication | What to do in a specific case |
| Edema | Evidence of a local allergic reaction. Shortness of breath may develop. | Take an anti-allergenic remedy and remove the toothpaste from the tooth in the doctor's office. |
| Redness of the gums | The carelessly applied drug got onto neighboring tissues. | You can wait until the doctor removes the medicine. |
| Black enamel | The enamel layer was exposed to a killing compound. Without bleaching, the stains will be permanently fixed. | The doctor must promptly remove the product and treat the membrane with a special composition that restores the color of the tooth. |
| Weakness, poor health | Drowsiness, dyspeptic disorders, loss of strength, headaches are signs of poisoning that require urgent medical attention. | The dentist must urgently remove the paste and prescribe absorbents that cleanse the organs of toxins. |
The medicine is used strictly according to the norm, but for people with hypersensitivity to its components, this dose is enough to survive all the experiences associated with intoxication. Some patients are afraid to disturb the doctor and bravely wait for the scheduled visit. Such carelessness can sometimes cost not only the health of the tooth, but also the life of the patient.
How to help the victim
Although mild poisoning does not require hospital treatment, only a doctor can determine the severity of the problem. Therefore, first of all, you need to call an ambulance, and during its journey, independently help the victim:
- provide fresh air,
- give 1 glass or 1 liter of acidified water to drink (with 1 liter of vinegar or 3 g of citric acid) to cleanse the stomach,
- if there is hydrogen sulfide water in the house, give it a solution (100 ml) to neutralize the poison and transform it into a safe substance - arsenic sulfide,
- give sorbents (any except activated carbon, which is useless in this situation),
- in case of dehydration, unsolder the victim little by little, but often,
- If a toxic substance gets on your skin, wash it off with soapy water.
It is unlikely that in any home there will be drugs for every occasion, much less antidotes against arsenic. Therefore, all other actions will be carried out by doctors.
Food poisoning. Symptoms
Almost all food products pose potential dangers and health risks. In some products, the level of food additives - the so-called "yes" - is "off the charts", others are contaminated with pathogenic bacteria, and others were simply consumed unwashed. Food poisoning, unfortunately, is not uncommon these days. If the rules for preparing, processing, and storing food are not followed, food poisoning may occur.
Food poisoning in an adult (child) can be:
- microbial, that is, associated with pathogenic bacteria (salmonellosis);
- non-microbial - associated with poisons of animal or plant origin. For example, mushroom poisoning or poisoning with the Japanese delicacy - poisonous fugu fish.
Also, symptoms of food poisoning in adults occur when consuming foods containing pesticides and heavy metal salts.
By the way, poisoning from fish, seafood and caviar is far from rare, since the waters of the world’s oceans are polluted with salts of heavy metals and other man-made “chemicals”.
Food poisoning in an adult can be associated with banal laziness in preparing food at home and the habit of eating at various public catering establishments. Food poisoning in a child is most often associated with his “knowledge of the world”: often children put into their mouths everything that interests them as soon as their parents turn away.
The most common symptoms of food poisoning:
- nausea;
- vomiting of eaten food;
- diarrhea (diarrhea).
Mushroom poisoning also causes convulsions, hallucinations, jaundice, and pain in the right side.
When starting treatment at home, do not forget to rinse your stomach from poor-quality food and take Enterosgel sorbent, or better yet, do not take risks and consult a doctor!
Enterosgel and treatment of poisoning
The ability of carbon (coal) and silicon (clay) sorbents to absorb harmful substances has been known for a long time. Our ancestors intuitively understood that wounds heal better if they are sprinkled with ashes or covered with clay. Ashes and clay were used for stomach pain, fever and other illnesses.
However, the low absorbency and tablet forms of enterosorbents limited their medicinal properties until recently. The invention of Enterosgel, a new generation gel-like sorbent, at the end of the 20th century opened up additional opportunities for saving lives and treating patients with poisoning.
Enterosgel is especially effective for oral poisoning. Often, its timely use relieves all symptoms of food poisoning, since it absorbs and removes not only toxins, but also pathogenic bacteria, without disturbing the normal intestinal flora. Toxins do not have time to be absorbed into the blood, and bacteria do not have time to multiply and show aggression. Enterosgel removes from the gastrointestinal tract the products of secondary inflammation and intoxication that accompany poisoning with caustic substances.
No matter how the poison enters the body, it enters the intestinal lumen through the intestinal mucosa and is then reabsorbed. Therefore, Enterosgel is used for almost all types of acute poisoning.
Enterosgel is also used to prevent chronic poisoning in people employed in hazardous work.
Chlorine poisoning. Symptoms
Chlorine compounds are used in everyday life to clean surfaces, disinfect pool water and bleach laundry. Since chlorine has become firmly established in our daily lives, it is important to know what symptoms of poisoning with chlorine-containing substances may bother victims.
Dry cough, choking, sore throat, drooling, watery eyes, vomiting - these are the signs of chlorine poisoning.
Depending on the severity of intoxication in the body, chlorine poisoning can be mild, moderate and severe. Poisoning in children of any degree can only be treated in a hospital!
After carrying out the first emergency measures (gastric lavage and taking Enterosgel sorbent), the victim is hospitalized for medical care.
How to get rid of arsenic without medical help
If the timing of using the drug for therapeutic purposes has been missed, and it is not possible to visit the dentist’s office, you can try to extract the paste yourself.
- First of all, maintain hygiene: clean hands, clean teeth. Instruments also need to be disinfected. This could be tweezers, a large sewing (or medical) needle. It is best to boil them and keep them in vodka or potassium permanganate.
Alcohol for sterilizing needles
- It is convenient to remove the temporary filling at the mirror. The main thing is not to touch neighboring healthy tissues and not to go deeper into the dental cavity.
- Under the filling there will be a grayish paste. It must be removed carefully so as not to crumble and swallow arsenic.
- Then the tooth cavity is thoroughly rinsed with a solution of soda or chamomile infusion.
Chamomile infusion for mouth rinse after filling removal
- The tooth cavity is closed with a cotton swab.
After such self-help, you should visit the dentist as soon as possible, since the decaying nerve will contribute to the development of inflammatory processes. If the paste is left in the mouth longer than expected, the paste will destroy the periapical tissue adjacent to the pulp. The next stage will be the occurrence of periodontitis. After just 3 days, the tooth will begin to turn black and decay.
Don't put off visiting the dentist
How toxic is the drug?
Today in dentistry, arsenic paste is used strictly according to indications, since if recommendations are violated or individual characteristics are violated, its toxic properties still manifest themselves. If you take treatment carelessly, complications are possible:
- darkening of dentin;
- development of periodontitis;
- swelling of the pulp;
- necrosis of bone and periosteum;
- general intoxication of the body.
Periodontitis and pulpitis
Today, arsenic paste is contraindicated for pregnant women and children due to its high toxicity, since many new methods for treating pulpitis have been developed.
Arsenic is not used to treat the teeth of pregnant women
Pathological anatomy of arsenic poisoning and arsenic in forensic medicine
The pathological picture of acute M. poisoning depends on the chemical. properties of M. compounds and ways of penetration of poison into the body (oral, inhalation, transdermal).
In case of poisoning with arsenites and arsenates orally, during the first hours, swelling and congestion of the mucous membrane of the mouth, pharynx, esophagus, stomach and intestines, focal hemorrhages, superficial necrosis of the intestinal mucosa, sometimes ulceration, swelling and enlargement of lymph nodes, follicles (Peyer's patches) are observed. and lymph nodes of the mesentery, particles of poison are found on the mucous membranes. In severe cases, pathomorphology, the picture in the intestines resembles changes in cholera.
Rice. 1. Macroscopic specimen of a kidney in case of arsenous hydrogen poisoning: the renal pyramids (indicated by arrows) are dark colored due to the retention of pigmented waste in the distal sections of the renal tubules; a centimeter ruler is given below. Rice. 2. Microscopic specimen of the same kidney: a hemoglobin cylinder (indicated by an arrow) in the lumen of the renal tubule; X 200.
In case of poisoning with arsenous hydrogen by inhalation, intravascular hemolysis with jaundice and the appearance of a bronze tint of the skin, acute hemoglobinuric nephrosis, liver dystrophy, and hemolytic anemia are observed. Macroscopically, the kidneys reveal black-brown radial striations in the renal pyramids, caused by the retention of pigmented waste in the lumen of the distal sections of the renal tubules (color. Fig. 1). Histologically, coagulative necrosis of the epithelium of the renal tubules with its subsequent rejection and regeneration, hemoglobin casts in the lumen of the renal tubules are revealed in the kidneys (color. Fig. 2). Changes in the liver macroscopically correspond to the picture of yellow dystrophy. Histologically, steatosis and focal or diffuse centrilobular necrosis are revealed. Electron microscopy in the kidneys and liver, the earliest damage is found in the endothelium of the capillaries, swelling, destruction of cristae and deformation of mitochondria, expansion of the endoplasmic reticulum, rupture of cell membranes, and pyknosis of nuclei are noted. Initial damage to the nephrothelium is more pronounced in the apical sections. They are characterized by rupture of cell membranes, desquamation of microvilli and cell necrosis. In hepatocytes, there is an almost complete disappearance of glycogen, destruction of mitochondrial cristae, the appearance of myelin bodies in the dilated cisterns of the endoplasmic reticulum, an increase in free ribosomes, and rupture of cell membranes.
If death as a result of acute poisoning with Arsenic compounds occurred a few days after M. entered the body, then with forensic medical evidence. examination of the corpse reveals dystrophic changes in muscles and nerve endings, and plethora of the brain. At court medical examination of the corpse, the most pronounced changes are noted in yellow-kish. form of poisoning.
Judiciary-chem. proof of M.'s poisoning lies in the detection of M. in the mineralization of various tissues of internal organs, bones, hair, nails, etc. using widely used chemicals. reactions to M.—Marsh tests and reactions with silver diethyldithiocarbamate in pyridine. Since antimony (see) can also give a positive Marsh test, then to identify M. crystals of the substance forming a grayish-black mirror on the surface of the glass are scraped off and dissolved in a few drops of concentrated nitric acid, jnepe solution - place on a glass slide and add cesium chloride and potassium iodide. M, unlike antimony, forms complex crystals in the form of regular six-rayed stars, painted red; under the influence of pyridine, they dissolve, and yellow-green crystals of the pyridine complex of M. and cesium iodides are formed at the edges of the drop. M in the mineralizate is determined mainly colorimetrically in the form of an arsenic-molybdenum complex, which has a blue color. The sensitivity of the method is approx. 0.5 mg M. in the analyzed volume. In practice, forensic medicine examinations also use neutron activation analysis (see Activation analysis) for the detection and quantitative determination of M. in the body by the formation of its isotope 76As as a result of neutron irradiation of corresponding samples of M.-containing tissues.