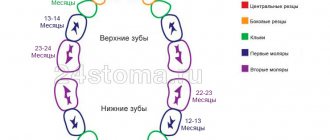
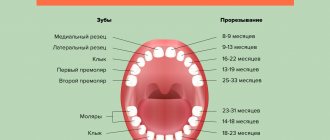
Formation of permanent teeth
Molars are formed from the epithelial dental plate. The appearance of their rudiments occurs only closer to the 5th month of fetal development inside the mother’s womb.
There are two groups of molars:
- Substitutes. This includes canines, incisors and premolars, which have temporary analogues.
- Additional. This group includes molars that do not have milk predecessors.
The growth of the primordial teeth of the replacement type occurs in the same alveolus as the temporary teeth; they are located behind the lingual surface of the primary teeth. Only after some time does the volume of bone tissue increase, ensuring their insulation.
Additional teeth are formed only after a year, since for this the jaw must reach the appropriate size.
Intramaxillary development
The permanent bite is formed on the basis of dental plates, which are formed inside the pits of stratified squamous epithelium. At approximately the 5th month of the child’s embryonic development, an enamel organ appears along the lower edge of the primordial teeth. As mesenchyme grows into it, dental papillae and sacs are formed.
In total, each child has 20 rudiments in two jaws for the development of replacement permanent teeth, 16 of which are present at the time of birth, and the remaining 4 appear at 1-5 years of life. Being in the same alveolus with the milk sacs, after a while they are separated from them by a bone septum and are placed in separate sockets. The dental plates grow in a posterior direction. The main dental tissues are actively developing - dentin, pulp, enamel, as well as blood vessels and nerve fibers.
At what age do they appear?
Statistics show that the beginning and completion of the change from temporary teeth to molars in most children occurs at approximately the same time. There are minimal differences only among children in different regions. The warmer the climate, the sooner the child will have permanent teeth.
The table shows age parameters that can be used to determine the approximate beginning of the eruption of molars according to several well-known authors.
| Set of teeth | Period of eruption of permanent teeth in children (in years) | ||
| according to Vinogradova | according to Lukomsky | according to Novak | |
| Central incisors | 5-6 | 6-9 | 6-9 |
| Lateral incisors | 7-9 | 7-10 | 7-10 |
| Fangs | 12-13 | 9-14 | 9-14 |
| First premolars | 9-11 | 9-13 | 9-13 |
| Second premolars | 9-11 | 9-15 | 10-14 |
| First molars | 4,5-7 | 7-8 | 5-8 |
| Second molars | 12-13 | 10-15 | 10-14 |
| Third molars | 18-25 | 15-24 | 18-20 |
The differences in the age at which permanent teeth appear depending on the author are due to the fact that they present the results of studies in different regions that were carried out with a serious difference in time (several decades).
Text of the book “Fundamentals of clinical dental morphology: a textbook”
As the enamel develops further, the enameloblasts decrease in size and move away from the dentin. Some enameloblasts die by apoptosis and are phagocytosed by neighboring cells. By the time teeth erupt, anameloblasts sharply decrease and are reduced, and the enamel is covered only with a thin shell - the secondary enamel cuticle, formed by cells of the outer enamel epithelium, collapsed pulp and an intermediate layer of the enamel organ, which covers the enamel and plays a protective role.
The process of enamel mineralization, starting in the area of the cutting edge and chewing tubercles of the teeth, then moves to other surfaces. The use of a new radiography method with primary direct image magnification [Belova N.A., 1981] showed that at 18–19 weeks of intrauterine development, signs of mineralization of the incisal edge and the surfaces of the incisor crowns by one third are clearly visible, as well as the beginning of calcification of the incisal edge of the canines and the vestibular medial cusp of the first molars.
At 24–25 weeks (6 months), mineralization of the incisors continues, the cutting edge of the canine is almost completely calcified, and points of calcification of the lingual medial tubercle of the first molars appear.
At 26 weeks (7 months), calcification of the incisors and canines continues, the vestibular cusps of the first molars have almost fused, and the first signs of mineralization of the apex of the distal vestibular cusp of the second molar are visible.
At 32 weeks (8 months), calcification of the incisors and canines continues; The vestibular cusps of the first molars merge.
At 36 weeks (9 months), all surfaces of the incisors are calcified (except for the cervical region), the vestibular cusps of the first molars are completely fused, the lingual cusps of the first molars are more clearly visible, and mineralization of the lingual distal cusp of the second molar is more intense.
After the birth of a child, the cervical area of the incisors becomes calcified; cervical region, vestibular and proximal surfaces of the canines, as well as the lingual surface of the first primary molar.
The final maturation of enamel (tertiary mineralization) occurs after tooth eruption, especially intensively during the first year of a child’s life. The main source of inorganic substances entering the enamel is saliva and, to some extent, dentin.
The study of the processes of development and calcification of enamel is not only of theoretical interest.
It is necessary for a correct understanding of the mechanism of development of a number of pathological processes that are observed in the enamel of developing or already completed teeth (enamel hypoplasia, caries). 6.2.3.
Development of the tooth root and cementum The development of the tooth root occurs already in the postnatal period, shortly before eruption. By that time, the crowns of baby teeth are mostly already formed. On top of the enamel layer of the tooth crown are the remains of the enamel organ in the form of an epithelial layer consisting of several rows of cells. This reduced enamel epithelium remains on the surface of the tooth crown until its eruption and, as some authors believe [48, 72], prevents the resorption of enamel from the connective tissue of the jaws or the deposition of cement on its surface.
These regressive changes in the enamel organ do not affect its edges, where the inner enamel cells merge into the outer enamel epithelium. The cells here actively proliferate, forming the epithelial (Hertwig) root sheath (vagina radicalis epithelialis), which plays an important role in the formation of tooth roots (Fig. 134). this vagina consists of inner and outer enamel organ cells closely adjacent to each other.
Rice. 134. Formation of the root of a growing tooth.
1 – epithelial root sheath; 2 – root dentin; 3 – root cement; 4 – dental pulp; 5 – epithelial diaphragm; 6 – epithelial bodies of Malasse; 7 – dental alveolus.
The inner enamel cells remain cubic in shape and do not develop into anameloblasts. The pulp of the enamel organ and its intermediate layer are absent. the epithelial vagina forms around the dental papilla, at its base, a kind of ring - the diaphragm (see Fig. 134, A) of the root sheath (diaphragma vaginae radicis), which gradually grows into the underlying ectomesenchyme, separating that part of it that will go to the formation of the tooth root .
The mesenchymal cells of the dental papilla, adjacent from the inside to the epithelial root sheath, turn into odontoblasts, which form root dentin (see Fig. 134, B). After the appearance of root dentin, the epithelial sheath grows with the cells of the dental sac, loses its continuity and is subsequently resorbed. Some vaginal cells may remain in the periodontium of an adult tooth in the form of residual epithelial Malasse bodies (see Fig. 134, B).
As a result of the disintegration of the epithelial root sheath, the ectomesenchymal cells of the dental sac come into direct contact with the root dentin, differentiating into cementoblasts, which begin to deposit cement on the surface of the root dentin.
The differentiation of ectomesenchymal cells of the dental follicle into active cementoblasts is accompanied by the development of granular endoplasmic reticulum, the Golgi complex, and a system of microbubbles and vesicles in the cells. First, cementoblasts synthesize internal collagen fibers of the extracellular matrix. The collagen released from the cells is further modified by specific non-collagen proteins, also derivatives of cementoblasts, into a form capable of calcification.
During cement mineralization, cementoblasts are constantly separated from the hardening front by a layer of non-calcified cement - cementoid, or precement. In addition to collagen, the cementum contains processes of cementoblasts. In some cases, cementoblasts are surrounded by their own excretory products, which harden, turning cementoblasts into cementocytes. The latter are an inactive form of dental follicle cells that lose organelles for protein synthesis and subsequently die, turning into scaly formations.
It is interesting to note that, unlike bone or dentin, the initial hardening of cementum does not depend on the activity of extracellular mineralized matrix vesicles, but occurs to some extent by the spread of hydroxyapatite from the dentin of the tooth root.
The rest of the dental sac, surrounding the developing tooth root, gives rise to the dense connective tissue of the pericement (periodontal). Bundles of collagen fibers of the pericementum with some of their ends are, as it were, soldered into the ground substance of the developing cement, and with the other ends they pass into the ground substance of the alveolar bone. As a result, the root is tightly strengthened in the dental alveolus.
In general, the formation of cement occurs according to the type of periosteal osteogenesis. The resulting cement is almost coarse-fibrous bone tissue in structure, covering the outside of the root dentin.
Exposure to unfavorable factors during cementogenesis can lead to hypercementosis (cement hyperplasia). The process can affect one or all teeth. Hypercementosis is also possible in certain areas of the root of one tooth.
In some cases, loose nodular thickenings of hardened cement form in the periodontal membrane. Such thickenings are known as cementicles. They are usually observed around degenerated epithelial remnants of Hertwig's vagina. With age, cementicles may adhere to the cementum, forming centers of localized excementosis.
After the formation of the root, the epithelial root sheath stops growing in depth, gradually closes in a ring shape and forms an opening at the apex of the tooth root, through which the vessels and nerves of the dental pulp pass. The initially wide apical opening of the root canal gradually narrows due to the deposition of new masses of dentin and cement.
Root development is more difficult in multi-rooted teeth. Depending on the type of tooth, the initially single and wide root canal of such teeth is divided into two or three canals. From the edges of the diaphragm of the epithelial root sheath, two (in the rudiments of the lower molars) or three (in the rudiments of the upper molars) epithelial outgrowths grow towards each other in a horizontal direction. The apices of these outgrowths eventually grow together and the initially single cervical opening is divided into two or three openings, according to the number of future roots. Their further development proceeds similarly to that of single-rooted teeth.
The growth of the roots of baby teeth and their subsequent resorption occur in a certain order. Thus, the roots of the central incisors and first molars develop during the first six months of a child’s life. Root growth continues until the age of 2–2.5 years, after which their gradual resorption occurs. The roots of the lateral incisors begin to develop during the first half of the year and grow until 2 years. Their resorption begins at the age of 5–6 years. The roots of the fangs develop in the period from 1 year to 3.5 years, and are resorbed in the 5th year.
The development of the roots of second molars occurs within 11.5 years.
By the age of 3, the roots are fully formed. Their resorption begins at the age of 5 years. 6.2.4.
Development of the pulp of primary teeth Simultaneously with the histogenetic processes of formation of dental tissues, differentiation of the mesenchymal elements of the dental papilla occurs in its central part.
Mesenchymal cells increase in size and begin to move away from each other due to the appearance of a basic amorphous substance between them. Gradually, the mesenchyme of the papilla is transformed into loose connective tissue, rich in fibroblasts, fibrocytes and macrophages, as well as blood vessels and nerves. By the time the tooth erupts, the mesenchyme of the papilla is almost completely differentiated into the pulp of the dental organ, although the final restructuring of the tissue elements of the pulp is completed in the first years of life. 6.2.5.
Eruption of baby teeth Eruption of a tooth is the process of its movement from its origin and development within the jaw until a crown appears in the oral cavity. This tooth movement is a complex, multifunctional stage in tooth development, as it is accompanied by coordinated changes in the growth of teeth, alveolar processes and the jaws themselves. During the period of eruption, developing teeth make movements in various directions, among which the following can be distinguished:
– vertical (axial) movement in the direction of the long axis of the tooth;
– movement in the distal, mesial, lingual or vestibular direction (change in position in the jaw);
– wedging (lateral movement);
– rotation – movement around the longitudinal axis of the tooth.
All these movements make it possible to maintain constant the relationship of the tooth germs to the edge of the developing alveolar process of the jaw, which is a necessary condition for the successful completion of tooth eruption. During eruption, the tooth makes a significant journey in the jaw, during which the following are observed:
– changes in the tissues surrounding the tooth;
– tooth root development;
– restructuring of the alveolar bone;
– development and restructuring of the periodontium.
In the connective tissue of the gums, which lies in the path of movement of the erupting tooth, regressive changes occur due to the pressure of the tooth on the tissue. These changes cause ischemia (malnutrition) and subsequent tissue atrophy and resorption. The reduced enamel epithelium covering the tooth crown (formed by the outer and intermediate layers of the enamel organ, as well as enameloblasts that have completed the production of enamel) secretes enzymes that also contribute to the destruction of the connective tissue separating it from the epithelium of the oral cavity. Approaching the epithelium lining the oral cavity, the enamel epithelium proliferates and subsequently merges with it. The epithelium of the oral cavity above the crown of the tooth degenerates and through the resulting hole the crown erupts into the oral cavity, while there is no bleeding, since the crown moves through the canal lined with epithelium (Fig. 135).
Having penetrated the oral cavity, the crown continues to erupt until it takes its final position in the chewing plane, meeting the crown of its antagonist.
The reduced enamel epithelium remains attached to the enamel of the unerupted portion of the crown, where it is called the primary attachment epithelium. It is subsequently replaced by secondary attachment epithelium, which is part of the gingival epithelium.
Rice. 135. Eruption of baby teeth.
1 – epithelium of the oral cavity; 2 – tooth cuticle; 3 – erupting tooth; 4 – gingival epithelium; 5 – dental alveolus.
Regressive changes in the connective tissue surrounding the erupting tooth occur simultaneously with the intensive development of its root due to odontoblasts producing dentin and ectomesenchymal cells of the dental sac, differentiating into cementoblasts that deposit cement on top of the root dentin. At the same time, growth of periodontal fibers and restructuring of the alveolar bone are observed. Intense deposition of bone tissue in some areas is combined with its active resorption in others. The severity of these changes varies throughout the entire period of eruption and is not the same in different teeth.
Deposition of bone tissue occurs, as a rule, in those areas of the bone socket from which the tooth is displaced, and in the areas towards which the tooth migrates, resorption is observed. Resorption of bone tissue makes room for the growing tooth, reducing resistance to its movement. Bone deposition usually manifests itself as the formation of bony trabeculae separated by wide spaces.
In the incisors, areas of increased deposition of bone beams are the bottom and lingual surface of the alveoli, which indicates a displacement of these teeth towards the lips during eruption.
Bone tissue in premolars and molars is deposited on the bottom and distal walls of the dental cell, which indicates their additional medial displacement during axial movement during eruption.
In multi-rooted teeth, the deposition of bone beams occurs most intensively in the area of the future interradicular septum.
The main periods of laying, formation and eruption of milk teeth are reflected in the table. 3.
Table 3
Timing of formation, formation and eruption of baby teeth in humans
Note:
i/w – period of intrauterine life.
6.2.6.
Development and eruption of permanent teeth The development of permanent teeth occurs in the same way as the development of primary teeth, but more slowly (Table 4).
Table 4
Timing of the formation, formation and eruption of permanent teeth in humans
Note:
i/w – period of intrauterine life.
The source of their formation is the same dental plate from which the rudiments of milk teeth developed. Starting from the 57th month of prenatal development, along the free edge of the dental plate (some scientists propose to call these sections of the dental plate the replacement dental plate), behind each baby tooth germ, on the lingual side, the enamel organs of the anterior permanent teeth are formed: incisors, canines and premolars. Since there are no premolars in the child’s primary dentition, the primary molars are subsequently replaced by permanent premolars.
In the prenatal period, the rudiments of the incisors (6-7th month) and canines (7-7.5 months), and then the premolars (7.5 months), are formed first from the group of anterior permanent teeth in the prenatal period. As a result, 10 rudiments of replacement permanent teeth appear in each jaw of the embryo.
Initially, the rudiments of these teeth lie in the bone alveoli common with the rudiments of milk teeth, but then a bone septum grows between them and a gradual separation of the cells of milk and permanent teeth occurs.
At the same time, the dental plate continues to grow posteriorly in each jaw and along its edge the enamel organs of large molars are formed (it is believed that these teeth should also be embryologically classified as the generation of milk teeth, rather than permanent teeth).
The earliest appearance of the first molar rudiment is in the 56th month of intrauterine life. The formation of the remaining molars occurs after birth: the rudiment of the second molar appears in the 5-6th month of a child’s life, and the rudiment of the third molar - in the 5-6th year of life, which is probably associated with the growth and elongation of the jaws. As they grow, the dental plates lengthen and grow posteriorly, the ends of which are displaced in the lower jaw into its branch, and in the upper jaw into the maxillary tubercle. This is where the enamel organs of the second and third permanent molars are formed. Only after this the dental plate gradually resorbs and disappears.
The very development of permanent teeth and differentiation of dental tissues occur in the same way as milk teeth. Ectomesenchyme grows into the enamel organs of permanent teeth from below and a dental papilla is formed. In the circumference of the germ of permanent teeth, a dental sac is formed from the ectomesenchyme and the further development of permanent teeth proceeds in the same way as milk teeth. The difference lies only in the time of passage of individual stages and the longer development of permanent teeth (about 10 years), especially large molars. Thus, during the period when milk teeth go through the last stages of their development, the jaws already contain the laying of permanent teeth that are at earlier stages. Therefore, in a child aged 3 to 6–7 years, an X-ray examination can reveal from 48 to 52 teeth in both jaws, of which 20 (baby teeth) perform their function, and the rest are still at various stages of development.
Calcification of the rudiments of permanent teeth is not observed before birth, with the exception of the first molar. A point of calcification in one of the cusps of the crown of this tooth appears on
9th month of intrauterine life. Complete crown formation occurs by two years. Calcification of the incisors begins in a child by 3–4 months. Complete crown formation occurs in the central incisors at 2–2.5 years, and in the lateral incisors by 3 years. Fangs begin to calcify at 4–5 months of age, and crown formation ends at 4–4.5 years. The process of mineralization of small molars begins at the end of the 2nd – beginning of the 3rd year of a child’s life and ends by 6–7 years. The beginning of calcification of the second molars dates back to 45 years. The third permanent molars calcify by 1315.
The formation of the roots of the medial incisors begins at the age of 3–3.5 years, the lateral incisors at the age of 4–4.5 years, the canines at the age of 5.5–6 years, and the first molar at the age of 4–6 years. The roots of the medial incisors reach full development at the 9-10th year, the lateral ones - at
10–11 years, canines by 12–15 years; premolars - by 12-14 years, first molars - at 9-10 years, second - at the age of 14-16 years, third molar - from 18 to 25 years.
At the age of 6–8 years, permanent teeth begin to erupt. The first large molar erupts first, then the medial and lateral incisors appear, then (from 9 to 14 years) the premolars and canines, as well as the second molar and the third molar later (at the age of 18–25 years, sometimes later).
The process of eruption of permanent replacement teeth is accompanied by the loss of milk teeth. During this period, resorption occurs first of the dental alveoli, and subsequently of the roots of the teeth (Fig. 136).
Rice. 136. Development and eruption of primary and permanent teeth.
1 – primary incisor; 2 – gums; 3 – rudiment of the permanent incisor; 4 – bone alveolus.
As the permanent tooth begins its vertical movement in the jaw, it puts increasing pressure on the alveolar bone surrounding the primary tooth. In the connective tissue separating the alveolus of a baby tooth from the crown of a permanent tooth, giant multinucleated cells—osteoclasts—differentiate, which actively resorb bone tissue.
After the destruction of the alveoli in the process of ongoing vertical movement, the permanent tooth begins to exert pressure now on the root of the baby tooth. Around the latter, osteoclasts (odontoclasts) also differentiate from the connective tissue, which actively resorb the tissues of the baby tooth.
Odontoclasts are located on the surface of the tooth root in wide lacunae. They are large in size, with numerous outgrowths of the cytoplasm on the side facing the root, containing a large number of lysosomes and mitochondria.
The destruction of primary tooth root tissues (dentin and cementum) by odontoclasts includes their demineralization, phagocytosis of areas and their intracellular digestion. The localization of the initial sites of root resorption depends on the location of the anlage of the replacement teeth: in primary incisors and canines it begins in the apical region on the lingual side, in primary molars - on the interradicular surface. Cells of the pulp of the baby tooth also participate in this process, from which odontoclasts also differentiate, destroying predentin and dentin from the pulp side of the tooth.
The process of resorption of the roots of baby teeth begins long before the eruption of the corresponding permanent teeth and proceeds very slowly and in waves: periods of increased resorption are followed by periods of rest. During the latter period, cementoblasts or fibroblasts appear in the tissues, the activity of which leads to the partial development of reparative processes - the deposition of cement or bone-like tissue on the surface of destroyed dentin.
The amount of resorbed tissue is usually greater than that of newly formed tissue. Therefore, the process of destruction of the baby tooth progresses, which ultimately leads to the loss of connection between the tooth and the alveolar wall and the empty crown being pushed into the oral cavity. This crown usually falls out under the influence of a growing permanent tooth, chewing forces, or a slight mechanical impact on it.
Loss of primary teeth typically occurs symmetrically on the right and left sides of each jaw, and occurs faster in girls than in boys. With the exception of second molars, primary teeth in the lower jaw fall out earlier than the corresponding teeth in the upper jaw.
The eruption mechanism is generally similar in permanent and baby teeth. According to a number of authors, the eruption of permanent replacement teeth is facilitated by the presence of a special anatomical structure - a conductive channel, or cord. The anlage of such a permanent tooth is initially located, as noted, in the common bone alveolus with the milk tooth. Subsequently, it is completely surrounded by alveolar bone, with the exception of a small narrow canal containing the remains of the dental plate and connective tissue. Together, these structures are called the conductive cord, which contributes to the directional movement of the permanent tooth during its eruption.
The eruption of additional permanent teeth (large molars) is not accompanied by destruction of the roots of milk teeth, since they do not have predecessors, but occurs in the same way as ordinary milk teeth.
The first permanent teeth to erupt are the first molars, usually on the lower jaw first; behind them are the medial incisors, lateral incisors, first premolars, second premolars and canines, second molars and finally third molars (wisdom teeth).
Full development of the roots of these teeth is completed within 2–3 years after eruption. Thus, the process of replacing baby teeth with permanent ones lasts for 14–17 years. Therefore, at approximately 5–6 years of age (from 6 to 12 years), a mixed set of teeth is observed in the child’s jaws.
The views on the explanation of the biomechanics of teething by various authors are ambiguous. There are a number of theories of teething: the theory of tooth root growth, the theory of increasing hydrostatic pressure in the dental pulp and in the periapical region, the theory of bone tissue remodeling, the theory of periodontal traction, etc. [5].
According to the root growth theory,
the process of eruption is the result of the tooth being “pushed” out of the alveolus by the growing root. From the perspective of this theory, the eruption of a tooth with an unformed root, the complexity of the trajectory of the tooth before eruption begins, as well as the lack of resorption of the bone tissue of the dental alveolus, which opposes the root apex, are inexplicable.
The theory of increasing hydrostatic pressure in the dental pulp and in the root apex area
involves the participation in the mechanisms of tooth eruption in the process of increasing the volume of the pulp in the direction of eruption, as well as increasing blood supply and vascular permeability in the area of the root apex.
According to the theory of bone tissue remodeling
The direction of tooth movement during eruption is determined by the direction of bone tissue growth in the area of the bottom of the dental alveoli and its resorption from the opposite side. From the standpoint of this theory, it is difficult to exclude the transformation of bone tissue as a consequence of tooth eruption itself.
Periodontal traction theory
provides for the dependence of the eruption process primarily on the formation of periodontium, the development of which is accompanied by a stop in tooth eruption.
Apparently, each of the existing theories of tooth eruption reflects one of the aspects of this complex process.
Teething sequence
Almost all parents believe that the first molars should be incisors, which replace temporary elements of the dentition. But this opinion is wrong. Even before baby teeth fall out, at the age of 5-6 years, children receive their first molars, which are not on the list of primary teeth.
After this, the sequence of formation of a permanent bite is almost no different from the order of eruption of primary teeth:
- the lower and upper central incisors grow;
- lateral incisors appear on both jaws;
- lower and upper first premolars;
- fangs;
- upper and lower second premolars;
- second and third molars (you must understand that the so-called “wisdom teeth” sometimes do not penetrate the surface of the gums at all).
Teeth cutting in this order does not happen just like that, because it ideally corresponds to the speed of development and formation of the maxillofacial system. If the optimal sequence is followed, correct bite development occurs.
Competition "bio/mol/text"-2017
This work was published in the “Free Topic” category of the “bio/mol/text” competition 2017.
The general sponsor of the competition is: the largest supplier of equipment, reagents and consumables for biological research and production.
The sponsor of the audience award and partner of the “Biomedicine Today and Tomorrow” nomination was.
"Book" sponsor of the competition - "Alpina Non-Fiction"
...They say evil has no face. Indeed, no feelings were reflected on his face. There was not a glimmer of sympathy on him, but the pain was simply unbearable. Can't he see the horror in my eyes and the panic on my face? He calmly, one might say, carried out his dirty work professionally, and at the end he politely said: “Rinse your mouth, please...”
This is how Dan Andrews describes a visit to the dentist in his short story “Wretched.” Indeed, since childhood, we have been in incredible awe of such specialists as dentists. Parents do everything they can to force their children to at least go to the doctor’s office, trying not to think about what awaits them next. And sometimes an adult’s soul sinks at the sight of numerous tools. Sometimes just the sight of a dental clinic is enough to do this.
As a result, the state of the oral cavity and dental hard tissues around the world does not inspire hope for a future without caries. Despite advances in dental treatment, tooth loss remains one of the most significant problems. Thus, according to WHO, the main causes of tooth loss are caries and periodontitis. Complete tooth loss is particularly common among older people. Globally, approximately 30% of people aged 65–74 years are missing teeth due to inflammatory periodontal diseases and pathology of dental hard tissues [1].
Therefore, it is not surprising that the state of the oral cavity of the population not only in Russia, but also in the world represents a serious problem, offering opportunities both for study and, more importantly, for the search for new treatment methods. One of them was tissue engineering , an interdisciplinary branch whose goal is to create biological substitutes that restore and maintain the functions of a tissue or organ. The fairly high efficiency of tissue engineering methods and their potential have attracted the attention of many scientists. This also contributes to their unfading popularity in various fields of medicine to this day.
Duration of growth
Typically, children say goodbye to their last temporary teeth at about 12-13 years of age, although the roots of baby teeth dissolve even earlier. By that time, the oral cavity already has molars that are actively growing, and their roots are at different stages of formation.
It is necessary to understand the approximate timing of growth and root formation in case of deviations. It is these indicators that are taken into account when choosing a treatment method.
Experts distinguish two main stages of development of the roots of permanent teeth:
- Stage of unformed apex.
- Unclosed apex stage.
At the first stage, the length of the root becomes maximum, but its walls are parallel to each other. The channel is of sufficient width; it ends in the area of the future apex with a bell. The periodontal gap can be seen only on the sides of the tooth root.
At the next stage, there is a gradual formation of the upper part of the root, the convergence of the root walls and the release of the periodontal fissure, the apical region of which is slightly enlarged.
Formation of periodontium and roots
This stage of development begins after the eruption of permanent teeth and usually lasts for 3.5-5 years. It includes the development processes of surrounding tissues, as well as the root system. At the same time, the crown of permanent teeth at this stage is almost completely formed and is ready for active mineralization after eruption.
The period of development of the root part and periodontal tissues is divided into the following stages:
- active growth of the tooth root in length;
- development and strengthening of the root apex;
- uncovered root tip;
- formation and strengthening of periodontium;
- completion of periodontal and root development.
The completion of tooth root formation occurs at approximately this age:
| Teeth | Upper jaw, age | Lower jaw, age |
| Central incisors | 9-13 | 7-11 |
| Lateral incisors | 9-12 | 8-11 |
| Fangs | 9-12 | 9-12 |
| First premolars | 11-13 | 11-13 |
| Second premolars | 11-13 | 11-13 |
| First molars | 9-12 | 9-12 |
| Second molars | 14-15 | 14-15 |
Since the eruption of third molars does not occur at a specific time, it is impossible to establish a clear age at which their roots are formed.
X-ray results confirm the completion of the process of tooth root formation. The key signs are the absence of an opening at the apex, as well as a pronounced periodontal contour.
Thus, completion of dental growth, including full maturation, usually occurs only between the ages of 15 and 18 years. It is at this time that the maxillofacial apparatus already has the same dimensions as in adults.
Non-Hong Kong "Triad"
Now that we know so much about the origin and development of the tooth, we can move directly to the topic of interest to us - tissue engineering.
Tissue engineering is a set of methods and procedures aimed at the regeneration of biological tissues. It includes a triad of main elements (Fig. 4): stem cells, extracellular matrix or scaffold, growth factors and signaling pathways [10].
Figure 4. Tissue engineering triad. The basis of the tissue engineering triad is stem cells, growth factors and extracellular matrix.
[10]
The goal of tissue engineering is to replace lost cells, tissues and organs, or promote their regeneration, or simply restore impaired function.
Today we hear and read a lot about stem cells. This is a hotly debated branch of science. The information that goes out to consumers, as a rule, is not always objective. What exactly are stem cells, and how and which of them can be used in dental tissue engineering?
Let's get acquainted: stem cells are undifferentiated embryonic or adult (postnatal) cells that are capable of going through a huge number of cell divisions while in an undifferentiated state, as well as forming intermediate cell types - precursors that can differentiate into various cells and create full-fledged tissues and organs (Fig. 5) [10], [11].
Figure 5. Classification of stem cells according to their ability to differentiate. Based on the scale of differentiation, stem cells are divided into totipotent, pluripotent, multipotent and unipotent. Totipotent cells are capable of differentiating into any cell type of an adult organism. Pluripotent cells can produce specialized cells of the three germ layers (ectoderm, endoderm and mesoderm), but not the entire organism. Multipotent cells produce a limited range of cell types. Unipotent cells are capable of differentiation into only one type of cell [13].
[11]
The first cell line of embryonic stem cells was isolated back in 1998 [12]. In fact, not so long ago, and from the point of view of the course of history one can say quite recently, but the progress is colossal [10].
Embryonic stem cells are isolated from the blastocyst during embryonic development. They give rise to three germ layers: ecto-, endo- and mesoderm. These cells are totipotent, meaning they can develop into each of the more than 200 cell types in the adult body [10].
There are currently 3 known sources of mammalian embryonic stem cells: cells isolated from the inner cell mass of the blastocyst; teratoma cells and primary germ cells of the embryo [10].
As was previously mentioned, stem cells are not only embryonic, but also postnatal. As for “adult” stem cells, they exist in the body in various tissues, including bone marrow, blood vessels, liver, skin, adipose tissue and dental tissue. They are localized in special niches where their proliferation, migration and life span are regulated. Postnatal stem cells are multipotent, meaning they give rise to only one type of cell.
Dental stem cells are a population of postnatal mesenchymal stem cells (MSCs) that have the ability to self-renew and differentiate [4], [14]. Depending on the location of the MSC depot (Fig. 6) [15], they are divided into:
- pulp stem cells;
- apical papilla stem cells;
- stem cells from extracted baby teeth;
- dental follicle progenitor cells;
- periodontal ligament stem cells;
- MSCs obtained from the alveolar process;
- MSCs of the gums;
- progenitor cells (MSCs aimed at differentiation only into a certain type of cell) of the tooth germ.
Figure 6. Dental stem cells. Schematic representation of sources of dental stem cells. For an explanation of the abbreviations, see the box below.
[15]
Abbreviations
WHO World Health Organization MSCs mesenchymal stem cells ECM extracellular matrix ABMSCs alveolar bone-derived mesenchymal stem cells BMP bone morphogenetic protein DFPCs dental follicle progenitor cells DPSCs dental pulp stem cells FGF fibroblast growth factor GMSCs gingival mesenchymal stem cells iPSCs induced pluripotent stem cells PDGF platelet derived growth factor PDLSCs periodontal ligament stem cells SCAP stem cells from the apical part of the human dental papilla SHEDs stem cells from human exfoliated deciduous teeth TGPCs tooth germ progenitor cells germ progenitor cells)
Let's look at some of them.
Pulp stem cells can be quite easily isolated from the pulp of extracted teeth. They represent a very attractive and promising source of autologous stem cells and can be used both for the regeneration of dentin, pulp and cement, and for the restoration of bone tissue [15]. In addition, they exhibit strong neuroregenerative activity, which is of particular value in the treatment of spinal cord injuries: pulp MSCs, in addition to suppressing the early inflammatory response, inhibit the apoptosis of neurons, astrocytes and oligodendrocytes after injury, which leads to the preservation of the nerve fiber and myelin sheath. They have also been found to promote the regeneration of severed axons. Thus, scientists hypothesize that pulp MSCs could provide significant therapeutic benefits in the treatment of spinal cord injury [16].
Stem cells from extracted primary teeth are a postnatal population of stem cells with high proliferative capacity, high viability, and the potential for multilineage differentiation (e.g., into osteoblasts, neuronal cells, and odontoblasts) [15].
Gum mesenchymal stem cells are ideal for restoring damaged periodontal tissue, muscles and even tendons. But it is not yet entirely clear whether they are capable of forming dentin and pulp cells [15].
Tooth germ progenitor cells are a relatively new population of stem cells that were discovered in the mesenchyme of the third molar germ at the bell stage. They show the same multilevel differentiation as other dental MSCs, including the ability to differentiate into adipocytes, osteoblasts, odontoblasts, chondrocytes and neurons, and can also differentiate into cells with the morphological, phenotypic and functional characteristics of hepatocytes. Hence, it is assumed that this type of stem cells can be used in the future to treat liver diseases [15].
Thus, each type of dental stem cells has its own characteristics and areas of application not only in dentistry, but also in other areas of medicine.
In addition to the MSCs described above, induced pluripotent stem cells (iPSCs) derived from somatic cells are also used in tissue engineering. They were first discussed in 2006, when Japanese scientists Kazutoshi Takahashi and Shinya Yamanaka showed that somatic cells can be reprogrammed into iPSCs by increasing the expression of certain transcription factors (Oct3/4, Sox2 and Klf4) [17], [18]. These cells themselves are immunologically neutral and, just as importantly, do not raise the same ethical controversy as embryonic stem cells. However, viral agents were used to reprogram them, which could lead to the formation of neoplasms [19]. There were attempts to use chemical molecules instead of viruses [20], but, unfortunately, the percentage of successful reprogramming turned out to be small. New methods for obtaining iPSCs are now being developed, since their application looks quite attractive and very promising.
What does it cost us to build a tooth?
To use stem cells in tissue engineering, the presence of a scaffold and growth factors is required (Fig. 7). An ideal scaffold should support cell attachment, migration, proliferation, and spatial organization.
Figure 7. What does it cost us to build a tooth?
website dentistry.tamhsc.edu
Basically, a scaffold as a suitable matrix for tissue reconstruction should meet the following requirements [21]:
- Ease of use.
- The presence of pores of a certain shape and size for the diffusion of cells, growth factors, nutrients and removal of waste products.
- The ability to biodegrade, which occurs at a certain time without releasing toxins.
- Biocompatibility with body tissues.
- Low immunogenicity.
- Ability to be replaced by regenerating tissue and vascularization.
- Good physical and mechanical properties.
The materials used to form scaffolds are divided into natural and synthetic (Fig. [22]. Bioactive glass, polylactic acid, various composites (multicomponent materials based on a matrix based on metal, polymer or ceramic) - all these are synthetic materials. Despite the fact that these materials make it possible to produce scaffolds of the required shape, their use is very limited due to unsatisfactory biocompatibility and toxicity. Among the biomaterials (natural materials) used to create scaffolds, collagen, chitosan, and hyaluronic acid can be distinguished. They consist of macromolecules that They are also part of the extracellular matrix, therefore they are biocompatible and highly biodegradable, but they are less durable and can cause rejection reactions [21].
[22]. Bioactive glass, polylactic acid, various composites (multicomponent materials based on a matrix based on metal, polymer or ceramic) - all these are synthetic materials. Despite the fact that these materials make it possible to produce scaffolds of the required shape, their use is very limited due to unsatisfactory biocompatibility and toxicity. Among the biomaterials (natural materials) used to create scaffolds, collagen, chitosan, and hyaluronic acid can be distinguished. They consist of macromolecules that They are also part of the extracellular matrix, therefore they are biocompatible and highly biodegradable, but they are less durable and can cause rejection reactions [21].
Figure 8. 3D scaffold of mouse and human teeth. a — Lower central incisor of a mouse. b — Human lower first molar. 3D reconstruction and bioprinting were used. Material: hydroxyapatite and polycaprolactone. Microchannels (d = 200 nm) into which MSCs and growth factors are introduced ( c and d ) are visualized.
[22]
The most suitable scaffold that meets most requirements is either a scaffold derived from extracellular matrix ( ECM scaffold ) or its analogue. Due to their identity with the extracellular matrix, such scaffolds are able to provide the best interaction with cells and growth factors. Dental MSCs, such as pulp and periodontal stem cells, when cultivated in ECM scaffolds, underwent differentiation in the odontogenic direction. After implantation of this scaffold, the pulp was formed [10], [23].
In addition to the scaffold and stem cells, a link is needed that connects them, which would regulate tissue growth. These can be growth factors, certain genes, and interfering RNAs [7].
Growth factors are peptide molecules that transmit signals to control cellular behavior and interact with specific receptors on the surface of cells [24]. They provide interconnection and interaction between cells and the extracellular matrix. Following cell damage, the secretion of growth factors begins, which subsequently trigger the processes of regeneration and angiogenesis. An example of the “work” of growth factors in a tooth is the formation of secondary and tertiary dentin, which occurs when the carious cavity is close to the dental pulp or when teeth are subject to increased abrasion. Key growth factors during tooth development include bone morphogenetic protein (BMP), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). They are primarily used in dental tissue engineering [25–27]. Both cells and nanoparticles, as well as the scaffold itself, can be used to deliver growth factors.
Forward to the future!
Of course, there is no doubt that dental bioengineering will soon become an integral part of standard protocols for the treatment of dental lesions. It is possible that regenerative dentistry techniques will allow us to create a complete dentogingival complex. It is important to remember that methods developed in accordance with the requirements and objectives of dental bioengineering will be able to spur the development of new approaches to the regeneration of other tissues and organs and thus contribute to progress not only in dentistry, but also in the field of regenerative medicine in general. Well, forward to the future!