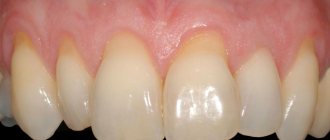
Osteoplasty in dentistry is the augmentation of bone tissue necessary for successful implantation. After a person has a tooth removed, the hard tissue tends to dissolve, and the new artificial tooth must be held securely in the jaw. Bone augmentation for dental implantation successfully solves this problem, but requires additional costs and more time (than, for example, with one-stage implantation, when the tissue volume is sufficient).
Osteoplasty – what is it and why is it needed?
When introducing a titanium root, it is very important that it is surrounded on all sides by bone. If there is a deficiency of tissue, the titanium pin will be rejected. Moreover, if the root is installed in the upper jaw, there is a risk of touching the maxillary sinuses, which can lead to serious ENT diseases. Also, if there is insufficient bone, the implant will become loose and sink below the gum, which will affect the aesthetics of the prosthesis and the overall picture.
Osteoplasty is recommended in the following cases:
- if it is necessary to install a root in an area where a tooth was removed several years (or months) ago;
- if the patient has anatomically large maxillary sinuses;
- when physiologically a person has a minimum volume of hard tissue.
The operation is not performed if:
- the patient has chronic ENT diseases (sinusitis, sinusitis, rhinitis);
- lack of calcium in the body;
- there are pathologies in the structure of the respiratory system;
- oncology;
- polyps and other formations in the nose;
- diabetes.
Also, osteoplasty is not performed on pregnant and lactating women. Otherwise, the operation is done relatively quickly and with a guarantee.
In many cases, patients are recommended to take a course of hormonal medications and antibiotics before surgery in order to neutralize edema and possible inflammation.
Splitting of the alveolar process -
Used in horizontal bone resorption to increase the thickness of the alveolar process. Can be performed on both the lower and upper jaws. It must be said that this is the most effective method of expanding the alveolar process today, which also has a low cost (it does not require expensive bone materials and membranes). There are several varieties of such splitting, but we will especially focus on the “Split-Control” technique, which allows for simultaneous expansion and installation of implants.
The content of the “Split-Control” technique (Fig. 5-10) - after detachment of the mucoperiosteal flaps (gum) in the center of the alveolar ridge, a cut is made with a milling cutter or other special tools to the height of the future implant (Fig. 6). Next, a pilot drill is used to mark a hole for the implant(s) and spreaders are screwed into the prepared holes (Fig. 7). By using different sizes of spreaders from smaller to larger, you can increase the width of the ridge and immediately install the implant.
There is always a gap left on the sides of the implant, which is filled with bone material, which, if necessary, can be applied in excess to the outside of the alveolar process, covering it all with a special resorbable membrane (Fig. 9). After which the wound is sutured, and we wait for osseointegration of the implant within 3-4 months.
Bone grafting of the lower jaw (splitting method) –
Advantages of the technique –
- Firstly , thanks to the splitting of the ridge, we get a bone defect that has bone walls on all sides (except on top).
Thanks to this, rapid and high-quality osteogenesis (new bone formation) occurs, because the spongy bone deep in the alveolar process is rich in blood vessels, osteoblasts, mesenchymal cells, growth factors... Speaking of why it is much worse to increase the width of the bone not due to splitting (from within the alveolar process), and do this by externally attaching bone blocks or bone chips outside cortical plastic surgery of the alveolar process (24stoma.ru). The fact is that the outer cortical layer of bone is very dense and there are practically no blood vessels in it. Accordingly, the implanted bone material will take a very long time to grow blood vessels, bone formation will be slower, and there will be a greater risk of failure and complications of such bone grafting.
- Secondly , there is no need for expensive bone materials and membranes, again due to the fact that this is a three-wall defect inside the alveolar process, and not outside it. There are enough inexpensive materials, for example, the bone material "Osteodent-K" and the membrane "Osteodent-Barrier". But if you are principled, then you can also use expensive materials like “Bio-Oss”.
- Thirdly , installation of implants with this technique is possible in most cases immediately. If the implants are installed later, then only 3-4 months will have to pass between operations, which is significantly less compared to other bone grafting methods.
Splitting of the alveolar process: animation and video of the operation
Important: There are several types of splitting techniques. With “Split-Control”, the cutter makes only a cut along the crest of the alveolar process + a pair of vertical cuts to the thickness of the cortical plate. But there is a variation of this method, where an additional horizontal cut is made at the level of the tops of future implants, which leads to complete detachment of the bone block (vestibular cortical plate).
Then this block is fixed with screws, which often break it. With this modification of the technique, implants are not installed immediately, but after 3-4 months. In addition, it is quite traumatic and there is a greater risk of complications. This type of technique should only be used for the thinnest alveolar process (2 mm), but some doctors use it even in cases where this is not necessary.
Types of bone tissue in dentistry
- allograft is synthetic bone tissue in dentistry, which is created in the laboratory. This is an artificial material that does not always take root successfully;
- intraoral autograft – the patient’s own bone tissue, which was taken from other areas of the oral cavity. For example, from the farthest part of the dentition. This augmentation procedure is called autotransplantation;
- allogeneic bone material in surgical dentistry is human bone, the donor of which is another patient. High survival rates, but the patient may be confused that this material was taken from a cadaveric bone, although it was stored in sterile packaging;
- Xenograft is a bone tissue substitute in modern dentistry, a material of animal origin. Borrowed from cattle bones. One of the most popular materials for transplantation when a large volume of hard tissue is required;
- Hydroxyapatite granules are an artificial bone graft in dentistry.
An experienced dentist-implantologist will help you determine the specific type of bone material in dentistry, who will study the patient’s condition, the characteristics of the tissue and evaluate the future scope of work on restoring the dentition.
Reparative bone regeneration is the restoration after injury. This process is of great importance for practical medicine. Bone tissue is unique because bone is capable of completely restoring even significant defects, unlike all other tissues in which regeneration ends with the formation of a connective tissue scar or organ hypertrophy.
There is no single definition of the term “reparative regeneration”. Some authors argue that this is a type of physiological regeneration that occurs under conditions of extreme influences on the body, but is more intense. Others characterize it as a complex process that is caused by the destruction of bone structures, quantitatively exceeding the permissible limits of physiological regeneration and aimed at restoring integrity and function.
The processes of bone tissue regeneration in the maxillofacial region are a complex interweaving of a number of general effects at the systemic level and local changes in tissue metabolism, including changes at the molecular level. Reparative regeneration is based on cell differentiation, their proliferation, resorption of damaged tissue and new bone formation during remodeling, as well as the formation of an organic extracellular matrix with its subsequent mineralization.
Stages of healing of bone tissue of the maxillofacial area
When healing fractures, the following four stages are distinguished: reparative reaction, formation of fusions of bone fragments, fusion of bone fragments, functional restructuring of callus and fragments with the formation of an organ structure.
Currently, researchers distinguish several stages of reparative regeneration:
- Tissue catabolism, differentiation and proliferation of cellular elements.
- Formation and differentiation of new tissue structures.
- Formation of vascular formations to nourish new tissue.
- Restructuring of the primary regenerate.
In the first stage, the reparative reaction of bone, there are such phases as acute post-traumatic disruption of tissue blood supply, cell necrosis with disorganization of cellular structures, proliferation of non-viable mesenchymal stem cells and differentiation of proliferating cells towards the formation of bone differential (mesenchymal stem cell - preosteoblast - osteoblast - osteocyte ).
Acute post-traumatic disturbances of tissue blood supply in the fracture area are accompanied by rupture of the periosteum, endosteum, osteon canals, as well as bone marrow, vessels and nerves, muscle and connective tissue surrounding the bone. These processes are accompanied by hemorrhages, edema, macrophage infiltration and the development of diffuse ischemic degenerative-necrotic changes in tissues. This phase lasts about 6-18 hours from the moment of injury.
The second phase, lasting 10-24 hours after injury, is accompanied by disorganization of bone tissue structures, signs of necrosis and macrophage cell infiltration.
In the third phase, which occurs within 24-72 hours from the moment of fracture, against the background of restored blood supply, proliferation of mesenchymal stem cells, pericytes of the microvasculature, periosteum, endosteum with the formation of osteogenic tissue is observed. Depending on the location of the proliferating cells, periosteal (periosteal cells), endosteal (bone marrow cells) and intermediate (cells of the bone marrow and vessels of the central canals) bone formations are distinguished.
The fourth phase of the reparative reaction is accompanied by proliferation and differentiation of osteogenic cells into preosteoblasts and osteoblasts. They synthesize osteoid, which after mineralization turns into coarse fibrous bone tissue. Activation of osteoclasts is observed, which resorb necrotic bone tissue.
At the stage of formation of fusions of bone fragments, which occurs 3-5 days after the injury, bone regenerate, or callus, is formed, which spreads in the proximal and distal directions. After 2-6 weeks, this process leads to the fusion and consolidation of bone fragments.
The last stage of fracture healing is characterized by the formation of bone adhesions, which can be of several types. Primary bone fusion is accompanied by direct fusion of fragments at the level of the cortical layer with the formation of new osteons. Fibrous-cartilaginous fusion is characterized by the development of necrosis of bone and soft tissue in the fracture zone, a sluggish reparative reaction that develops at a considerable distance from the fracture site, and the predominant development of fibrous and, less commonly, cartilaginous tissue.
The existing fibrocartilaginous bone fusion as a result of prolonged ossification can be replaced by bone tissue, forming a secondary fusion of bone fragments. In the fourth stage, a functional restructuring of the callus and fused fragments occurs with the formation of the organ structure of the bone.
Cells and substances involved in reparative regeneration
of bone tissue
The process of regeneration of bone tissue in the maxillofacial area occurs with the participation of cells such as osteoblasts, osteoclasts or osteocytes . Below is a brief description of these cellular elements, indicating the features of their structure and functions.
Osteoblasts
are bone-forming cells that originate from mesenchymal stem cells. They are round in shape, 20-30 microns in size, with an eccentrically located core. These cells are located in the osteogenic layer of the periosteum and in the perivascular spaces of osteons.
There are four types of osteoblasts involved in the healing of bone tissue: preosteoblasts, proliferating functionally active osteoblasts, maturing osteoblasts with a hypertrophied endoplasmic reticulum, and differentiated low-active osteoblasts. The main part of osteoblasts synthesize bone tissue until their function stops, followed by their transformation into inactive cells.
These cells line the surface of the newly created bone tissue and connect to osteocytes through a system of tubules. A certain part of osteoblasts are encapsulated in the osteoid matrix and differentiate into osteocytes.
Osteoblasts have a developed Golgi apparatus and mitochondria, due to which they synthesize a huge amount of intercellular substance, in particular proteins and collagen fibers, which form the organic bone matrix - osteoid. Osteoblasts synthesize type I collagen from procollagen, which consists of one α2- and two α1-polypeptide chains forming a helical structure.
The procollagen molecule contains two terminal peptides: amino- and carboxy-terminal propeptides. After the secretion of procollagen by osteoblasts into the extracellular space, these two propeptides, under the influence of enzymes, are cleaved from procollagen, which is transformed into tropocollagen.
Free final propeptides of bone tissue enter the circulating blood, where their concentration can be determined by the enzyme immunoassay method. This analysis very accurately indicates the amount of type I collagen synthesized, with the level of amino-terminal propeptide most accurately characterizing its metabolism.
Osteoblasts also synthesize the non-collagenous fraction of bone matrix proteins, which play a leading role in its mineralization. These are osteocalcin, osteopontin, osteonectin, and bone sialoprotein.
Osteocalcin is the main non-collagenous bone protein that is involved in the binding of calcium and hydroxyapatite. It has a chemotactic effect on osteoclasts and is involved in bone resorption.
Osteonectin binds type I collagen and hydroxyapatite and takes part in the formation of the initial crystal (nucleation) during bone mineralization. Osteopontin also binds bone cells to hydroxyapatite in the extracellular space, but recent studies indicate the involvement of this protein in tumor metastasis by facilitating the adhesion of tumor cells during invasion.
Bone sialoprotein contains sialic acids. It is a calcium-binding glycoprotein that provides mineralization and stabilization of the collagen structure.
Osteocytes
, or “stellate cells,” have a large number of long and thin processes. They are divided into three types, the characteristics of which are briefly listed below:
- Type I osteocyte-producing cells synthesize bone matrix components.
- Type II osteocytes, mature or resorbing cells, are involved in the process of osteolysis.
- Type III osteocytes, degenerating cells, are located at the periphery of the osteon. As a result of their destruction, a significant amount of lysosomal enzymes is released and osteolysis of bone tissue occurs.
Osteocytes have receptors for parathyroid hormone, synthesize osteocalcin, matrix proteins and sclerotin, which is an inhibitor of the Wnt signal in osteoblasts. Their main function is the transmission of mechanical and chemical signaling to osteoblasts, integumentary cells and, through them, to osteoclasts. This circuit plays an important role in triggering bone remodeling processes under physiological and pathological conditions.
Osteoclasts
are multinucleated giant cells that provide resorption of bone matrix components. They are located on the surface of the bone in small depressions (erosive lacunae or Howship's lacunae). In places of contact with areas of bone resorption, the cytoplasm of osteoblasts forms outgrowths in the form of a corrugated border, which increases the area of contact with the bone and facilitates the entry of biologically active cellular products into the resorption zone.
Osteoclasts also secrete protons and proteolytic enzymes (cathepsin K, cysteine protease, matrix metalloproteinases) into the resorption zone and remove decay products into the surrounding space through the basolateral membrane. For such transport to occur, the presence of tartrate-resistant acid phosphatase (TRAP) is necessary. There are 5 types of acid phosphatases, which are produced by bone tissue, spleen, red blood cells, platelets and macrophages. All types of acid phosphatases are inhibited by tartrate, except for the fifth isoform, which is called TRKP-5.
Osteoclastogenesis,
osteoblastogenesis and bone remodeling
The following functional types of osteoclasts are distinguished: young, mature, functionally active and inactive, as well as dying. The source of origin of all osteoclasts is macrophage-monocyte cells of the bone marrow.
The RANK/RANKL/OPG system, which was discovered in 1997, plays an important role in osteoclastogenesis. RANKL, expressed on the surface of osteoblasts, binds to the RANK receptor, which is located on the membranes of osteoclast precursor cells. This triggers differentiation and activation of osteoclasts. The interaction of RANKL and RANK occurs in the presence of macrophage colony-stimulating factor (M-CSF), which, using high-affinity transmembrane receptors (c-fms), activates intracellular tyrosine kinase and stimulates the proliferation and differentiation of osteoclast precursors, mononuclear cells.
M-CSF activity has been shown to be significantly increased by exposure of osteoblasts to parathyroid hormone, vitamin D3, and tumor necrosis factor (TNF), but decreased by exposure to OPG and estrogens.
Osteoprotegerin is synthesized by osteoblasts, vascular endothelial stromal cells and B-lymphocytes. It is a soluble decoy receptor for RANKL that interferes with the interaction of RANKL and RANK, which impairs osteoclastogenesis and inhibits bone resorption. At the same time, interleukins (IL) -1,3,6,11, tumor necrosis factor-α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) and prostaglandins E2 (PG2) through the EP4 receptor are able to enhance the production RANKL by stromal cells of the bone environment, in particular osteoblasts, which stimulates osteoclastogenesis.
It was found that the production of osteoclasts is stimulated by parathyroid hormone. It is synthesized by the parathyroid glands. The hormone maintains calcium homeostasis by leaching from bone into the extracellular fluid and enhancing reabsorption in the renal tubules. This hormone activates the synthesis of acid phosphatase, lactate and citrate, and inhibits the synthesis of collagen and alkaline phosphatase.
With a sharp increase in the level of parathyroid hormone in the blood, activation of mature osteocytes and bone resorption (osteocytic osteolysis) are observed. With prolonged hypersecretion of parathyroid hormone, osteoclastogenesis increases and the activity of osteoblasts decreases, which also suppresses collagen synthesis. It has been proven that long-term administration of small doses of parathyroid hormone causes an anabolic effect, and this, on the contrary, promotes the maturation of cartilage tissue. It promotes the synthesis of 1,25-(OH)-2D3 in the kidneys under the influence of cyclic AMP from 25-(OH)-2D3.
Unlike parathyroid hormone, calcitonin, which is secreted in the interfollicular cells of the thyroid gland, reduces the number and activity of osteoclasts in bone tissue, suppresses osteocytic osteolysis and reduces the level of calcium in the blood. Calcitonin stimulates the maturation of chondrocytes in epiphyseal cartilage.
According to foreign studies in recent years, the leading role in the regulation of osteoblastogenesis belongs to bone morphogenetic proteins (BMP, bone morphogenetic protein). This is a group of signaling growth factors (cytokines) that actively stimulate the formation of enchondral bone tissue and regulate various cellular processes (proliferation, differentiation, apoptosis, chemotaxis, angiogenesis or production of extracellular matrix in tissues).
BMPs trigger the process of bone tissue formation through the expression of genes that regulate the processes of differentiation of mesenchymal stem cells with the subsequent formation of osteoblast cells. Dysregulation of the BMP signaling system is very often found in cancer.
Currently, 47 BMP subfamily proteins that interact with specific BMP receptors (BMPs) have been described. In the process of intercellular interactions in the bone tissue of the maxillofacial region, the following are especially important:
- BMP2: stimulate osteoblast differentiation.
- BMP3: promotes the formation of new bone tissue.
- BMP7: activate osteoblast differentiation and SMAD1 production.
- BMP8a: are directly involved in the development of bone and cartilage.
In practical medicine, BMP is used to stimulate bone healing processes. They are injected into a bone implant, from where they are delivered to fracture sites to improve osteogenesis.
Bone cells intensively secrete transforming growth factor β1 (TGFβ1) into the interosseous matrix, which activates the differentiation of mesenchymal stem cells according to the osteoblastic and chondral type, stimulates the processes of reparative bone regeneration, and enhances proliferation and collagen synthesis.
Processes associated with cell morphogenesis are also regulated by Wnt signaling. This process involves proteins discovered in the early 1980s as markers for certain types of cancer. They are also key regulators of the processes of remodeling and regeneration of bone tissue, differentiation of mesenchymal stem cells.
The canonical pathway of Wnt signaling in bone tissue is based on the stabilization of the cytoplasmic protein β-catenin. In the absence of a signal, it is inactive and quickly destroyed. When β-catenin is activated through Wnt, Wnt itself binds to cell surface receptors, which are the Friesland transmembrane protein. As a result, the destruction of β-catenin is inhibited, it accumulates in the cytoplasm and then penetrates into the nucleus. There it interacts with TCF/LEF proteins, which selectively bind to specific activator proteins and DNA sequences.
In this way, certain genes responsible for bone tissue regeneration are activated. Another, or non-canonical (β-catenin-independent) Wnt signaling pathway regulates cell polarity, stimulates calcium metabolism and cytoskeletal reorganization. Wnt signaling stimulates OPG production. It has been proven that at least 22 Wnt ligands are involved in the activation of Wnt signaling in humans.
An antagonist of the Wnt/β-catenin signaling pathway is sclerotin glycoprotein, which is produced by osteocytes of intact bone. It prevents MSC differentiation. When damage occurs to the bone tissue of the maxillofacial region, osteocytes transmit a signal to the integumentary cells that line the surface of the trabecula. Under the influence of prostaglandins and growth factors, which are released, the integumentary cells peel off from the surface of the bone, forming a specific canopy. The canopy cells combine with osteocytes and the capillary to form a bone remodeling compartment.
Recently, regulators of intercellular interactions between osteoblasts and osteoclasts, such as semaphorins and their receptors plexins, have been discovered. Semaphorins form a family of molecules consisting of 8 main classes of secretory and transmembrane proteins that are responsible for transmitting signals along axons. They regulate the growth, development and functioning of cells of the nervous, cardiovascular, immune, respiratory, and musculoskeletal systems.
In particular, Semaphorin 4D (Sema4D), which is a derivative of osteoclasts, acts on the membrane receptor Plexin-B1 on the surface of osteoblasts, suppressing the function of the latter. As a result, activation of bone tissue resorption is observed. Semaphorin 3B promotes the activation of osteoclasts, and semaphorin 3A, on the contrary, stimulates osteogenesis. Based on this, inhibition of semaphorins 4D and 3B is important for the treatment of osteopenia.
An important secretory derivative of osteoclasts is the SLIT3 protein. It stimulates the proliferation of osteoblast cells through activation of the β-catenin pathway. SLIT3 autocrine signaling inhibits bone resorption by suppressing preosteoclast differentiation. Experimental studies have shown that modified animals lacking SLIT3 or its receptor Robo 1 have low rates of bone formation and a high rate of bone resorption.
Practical assessment of bone tissue regeneration processes
The processes of bone tissue remodeling can be clearly assessed based on a comparative analysis of two groups of bone metabolism indicators - markers of bone resorption (hydroxyproline, hydroxyproline, calcium, breakdown products of type I collagen, pyridinoline and deoxypyridinoline, bone sialoprotein (BSP), tartrate-resistant acid phosphatase) and synthesis markers bones (osteocalcin, bone alkaline phosphatase, amino and carboxy-terminal fragments of type I procollagen, ACP, CCP).
Markers of bone tissue synthesis indirectly characterize the activity of osteoblasts. These cells take an active part in the formation of bone tissue, produce type I collagen plus other osteoid components, and participate in the mineralization of osteoid with hydroxyapatite.
The first biochemical marker of bone remodeling is the enzyme alkaline phosphatase, which was introduced into clinical practice in 1929 and is used today. There are 4 isoforms of this enzyme: bone, liver, intestinal and placental.
A laboratory indicator of osteoblast activity is bone alkaline phosphatase. This is a glycoprotein that takes part in the mineralization of the bone matrix. A simultaneous increase in ALP and parathyroid hormone may indicate the development of osteodystrophy with a high level of bone remodeling, and a decrease in these indicators may indicate an adynamic state of the bone tissue of the maxillofacial region.
The most informative marker of bone formation is osteocalcin. This non-collagenous bone matrix protein containing hydroxyapatite can be considered specific for bone tissue and dentin. It is synthesized primarily by osteoblasts and forms the extracellular matrix of bone.
A fraction of newly synthesized osteoclasts is released into the bloodstream, so this marker indicates the rate of bone tissue remodeling. An increased level of parathyroid hormone in the blood suppresses the activity of osteoblasts that produce osteoclasts and reduces the concentration of osteoclasts in the blood and bone tissue.
During the synthesis of type I collagen by osteoblasts, amino- and carboxy-terminal fragments are separated from type I procollagen as a result of the action of specific enzymes. The ratio between the amount of mature collagen deposited in the bone matrix and the number of terminal molecules that enter the bloodstream must be equal to one. Thanks to this, based on the indicators of ACF and CCP in the blood serum, one can judge the synthetic activity of osteoblasts in the synthesis of type I collagen.
The next group of bone remodeling indicators are markers of bone resorption. It should be noted that they change their level during the day, so they must be determined at the same time of day, preferably in the morning. Markers of bone tissue resorption include enzymes involved in the destruction of the bone matrix under the influence of osteoblasts and products of the destruction of type I collagen.
This is, for example, tartrate-resistant acid phosphatase (TRAP) - a metal-containing enzyme, one of 6 isoenzymes of acid phosphatase. It is secreted by osteoblasts into the extracellular environment during resorption. There are forms of TRAP-5a and 5b, but TRAP-5b is synthesized by osteoblasts, and TRAP-5a is of macrophage origin. TRAP-5b activity in blood plasma is an indicator of bone resorption processes.
Hydroxyproline is the main amino acid in collagen. It is not considered an autospecific marker of bone tissue, because only 50% of this amino acid is found in bones, and the remaining 50% is a component of other proteins, in particular elastin, acetylcholinesterase and complement factor C1q.
Most of it, after the destruction of collagen, is oxidized in the liver and only 15% is excreted in the urine. The content of hydroxyproline depends on the nature of the diet, the age of the person, the presence of a tumor with decay and demonstrates a circadian rhythm with a peak between 0.00 and 8.00 am.
14% of the amino acid composition of collagen is hydroxyproline. It is synthesized by osteoblasts and is also a marker of bone resorption. Nowadays, more specific markers of bone resorption are used, such as type I collagen breakdown products. These include pyridinoline (PID) and deoxypyridinoline (DPID). They are found in tissues containing type I, II, III collagen. PID is an important chemical compound, but DPID is found only in collagen, so it is evaluated as a selective bone marker.
Also widely used in medical practice is the determination of type I collagen peptides, NTX and CTX. The uniqueness of these indicators lies in the rapid increase in their level in diseases accompanied by high bone tissue resorption (for example, osteoporosis, bone metastases), as well as their rapid decrease (within several weeks) against the background of antiresorptive therapy.
The next marker of bone resorption is bone sialoprotein (BSP). During the process of osteolysis (physiological or pathological), the bone matrix is destroyed by osteoblast enzymes with the release of sialoprotein into the blood with a corresponding increase in laboratory BSP parameters. Bone sialoprotein is present only in mature osteoblasts and is not found in their precursors, so BSP may serve as a marker of late differentiation of bone cells.
Conclusion
Reparative regeneration of bone tissue in the maxillofacial region is associated with the activity of osteogenic cells. The local regulation of reparative regeneration is based on several signaling pathways, such as the RANK / RANKL / OPG molecular system, bone morphogenetic proteins, Wnt signaling and others.
Osteoblastogenesis and osteoclastogenesis characterize biochemical markers of bone remodeling, the determination of which allows one to determine the rate of metabolic processes in bone tissue, identify patients at increased risk and conduct an early assessment of the effectiveness of prescribed treatment, predict the risk of complications, diagnose the appearance of bone metastases in the early stages, etc.
Pros and cons of bone grafting during implantation
Competitive advantages of bone grafting:
- restoration of functions, even if the patient has lost a large amount of hard tissue;
- the appearance of the gum tissue and the oral cavity as a whole is normalized;
- restoration of chewing functions - all types of bone tissue in dentistry are adapted to heavy loads;
- the ability to restore teeth, even in the most advanced situations.
Disadvantages of bone grafting:
- rehabilitation takes several months;
- high risk of block rejection;
- pain during tissue engraftment;
- considerable cost of the operation.
Human embryo and sperm
It looks like a war of the worlds, but in fact, you have an egg in front of you 5 days after fertilization. Some sperm are still retained on its surface. The image was taken using a confocal microscope. The egg and sperm nuclei are purple, while the sperm flagella are green. The blue areas are nexuses, intercellular gap junctions that communicate between cells.
Consequences of atrophy
This disease can lead to certain consequences. The main thing is that it will become impossible to secure the implant and restore lost teeth. In addition, the patient can also observe negative changes in bone atrophy externally, that is, the shape and appearance of the face changes, and the functioning of many organs is disrupted.
Also, dental bone atrophy can lead to:
- formation of facial wrinkles in the lip area;
- the need for additional manipulations to increase bone volume before implantation;
- inability to chew food, which consequently leads to problems with the gastrointestinal tract;
- “recession” inside the lips;
- displacement towards the teeth, to the place where they are missing.
In addition, one of the important consequences of this disease is the increase in the cost of implantation. This is because a certain amount of bone will be required to secure the implants. If it is not enough, then you will need to build it up. And this implies the use of additional materials and medicines, and the work of a specialist.