- What are the benefits of fluoride?
- Why is fluoride deficiency dangerous?
- If there is too much fluoride in the body
- Fluoride products for teeth
- Water fluoridation
- Fluoride in toothpastes
- Can children have toothpaste with fluoride?
- Is there an alternative?
Most dental and oral care products contain fluoride. This is an important substance that helps strengthen enamel and prevents the spread of pathogenic microflora.
In the body, fluoride is contained in tooth enamel and bones in an amount of about 2.5–3 g. Our daily need for it is 1.5–5 mg. With a lack of this element, caries forms on the teeth. Therefore, in the USA and some European countries, fluoride is recommended for addition to drinking water and toothpastes.
Recently, articles have increasingly appeared that fluoride in toothpaste is harmful. Is it true? Let's try to figure it out.
What are the benefits of fluoride?
Fluoride performs important tasks in the body.
- Together with phosphorus and calcium, it participates in the formation and strengthening of bone tissue and tooth enamel
. - Promotes healthy nail and hair
. - Stimulates hematopoietic
: formation, development and maturation of red blood cells, platelets and leukocytes. - Strengthens the immune system
and maintains it at an appropriate level. - Removes salts of radionuclides and heavy metals
.
When used in oral care products, fluoride helps prevent dental problems and solve existing ones.
- Prevent tooth decay
. Fluoride penetrates the enamel structure, prevents caries and treats it at the white spot stage. - Protect against demineralization
. The combination of fluorine with hydroxyapatite (the main element of tooth enamel) forms fluorohydroxyapatite, a substance that restores it. This is how enamel remineralization occurs. - Protect against lactic acid
. Fluoride prevents bacteria from producing lactic acid. This reduces the growth of pathogenic microflora and gives the enamel additional protection.
Fluorine and its beneficial properties
- Strengthens enamel. The composition of enamel includes calcium, or, to be more precise, hydroxyapatite. When the latter reacts with fluorine, fluorapatite is formed - a strong compound that is especially resistant to the effects of cariogenic microorganisms. Regular use of toothpastes containing fluoride is a reliable means of caries prevention.
- Has a bactericidal effect. In simpler terms, fluoride kills organisms that cause tooth decay while also acting as a natural disinfectant.
- Prevents the formation of tartar. Since microorganisms do not stick to enamel treated with fluoride, tartar does not appear.
- Enhances the beneficial qualities of saliva. It has been practically established that fluoride, once in the oral cavity, increases the activity of the glands that secrete saliva, which plays an important role in the formation of caries. Saliva, which contains active calcium and phosphorus ions, intensively saturates tooth enamel. Under the influence of fluorine, it is saturated with mineral components that compensate for washed-out substances such as chlorine, magnesium, calcium apatite and many others. Saliva in combination with fluoride paste restores natural calcium loss, while reducing the risk of caries.
- Improves metabolism. It has been found that fluoride improves overall well-being and has a positive effect on metabolism. In the course of a long-term scientific study, it was found that pregnant women who include fluoride-containing products in their daily diet provide the child with stronger enamel without depressions and crevices.
If there is too much fluoride in the body
Fluoride is found not only in oral care products and tap water, but also in food.
The maximum permissible daily intake is 10 mg. Other causes of excess fluoride:
- chronic intoxication with fluoride compounds at work;
- improper regulation of fluoride metabolism in the body.
An excess of fluoride is more dangerous for the body than a deficiency, as it entails irreversible processes. First of all, teeth and bones are affected; metabolic disorders, deterioration of blood clotting, etc. may occur. In children, even before teething, endemic fluorosis develops - this is a chronic lesion of tooth enamel in the form of spots of various sizes, shapes and colors. After 10–20 years of excess fluoride in the body, bone fluorosis develops, which can develop into osteosclerosis, osteoporosis and osteosarcoma (malignant formation).
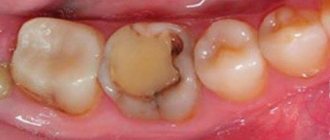
How does caries form?
Our teeth are covered in biofilm, more commonly referred to as plaque. It is home to millions of microorganisms. They are very happy when we eat carbohydrates and especially sugar, which they digest and at the same time release acid, as a result of which the pH in the oral cavity drops. What does falling pH mean for our teeth? Exactly the same thing that happens with scale (calcium carbonate) on a kitchen faucet: it is insidious in that it does not dissolve with water. BUT! Not if we treat the scale with acidic agents, such as vinegar or citric acid. Their low pH causes the mineral to dissolve!
The same thing happens to our teeth, which leads to the formation of a hole in the tooth - voila! Caries!
Tooth decay means that our teeth begin to dissolve. You could also say what is happening is DEMINERALIZATION. So, how can we overcome tooth decay? With the help of REMINERALIZATION! Everything is logical and simple!
Fluoride in toothpastes
The fluoride concentration in toothpaste is measured in ppm or percentage. The abbreviation ppm stands for parts per million and reflects the number of fluorine particles per million. If the tube states that the paste contains 900 ppm of fluoride, this means that 1 kg will contain 900 mg of this element.
The higher the fluoride concentration, the better the paste restores enamel. For prophylactic pastes it is 950–1150 ppm, for therapeutic pastes it is 1350–1500 ppm.
Fluorine in pastes can be found in various compounds: amino fluorides, sodium fluorides, sodium monofluorophosphates, tin fluoride.
| Component | Name on the tube | A comment |
| Sodium monofluorophosphate | Sodium monofluorophosphate | It is ineffective because due to the rate of decay it begins to act only after 3–4 minutes of cleaning. |
| Sodium fluoride | Sodium fluoride | Has a powerful antibacterial and restorative effect. The active substance reduces the ability of bacteria to convert sugar into acid, which destroys enamel. |
| Aminofluoride | Aminofluoride/Olaflur | Recognized as the most effective for caries prevention. Forms a protective film on the surface of the teeth, from which fluoride enters the enamel and strengthens it. |
| Tin fluoride | Stannous fluoride | It is proven effective, but has a side effect: it first lightens the restored areas of enamel, and then leads to their noticeable darkening. |
We recommend choosing pastes with sodium fluoride or amino fluoride.
Toothpastes with sodium fluoride
Toothpaste CURAPROX Enzycal 1450
miradent mirasensitive hap+ toothpaste for sensitive teeth
Blanx Advanced Whitening Toothpaste
Toothpastes with amino fluoride
miradent Mirafluor C toothpaste
Elgidium toothpaste Protection against caries
Toothpaste ROCS (ROCS) Teens The aroma of a sultry summer. Strawberries (from 8 to…
Fluoride in water - benefits and harms
When we talk about the benefits and harms of fluoride for teeth and the body as a whole, we must not forget about fluoridated water.
According to many experts, it is this that can most effectively restore the lack of substances in the body. On the other hand, fluoride is one of the most difficult elements to remove, and drinking fluoridated water contributes to its accumulation. Modern research suggests that if a liter of water contains more than 1 mg of fluoride, it can potentially be dangerous to the body. In past years, especially at the beginning of experiments with fluoridation, the dosage could be much more severe, causing different reactions in some people.
Many fluoridation opponents argue that in the 1950s, American courts were inundated with fluoride poisoning lawsuits. Be that as it may, the fact remains: today, water fluoridation is carried out in many countries and regions. The harm of fluorine in water is most often discussed when its increased concentration is detected or when toxic compounds are used, in particular fluoroaluminates.
Can children have toothpaste with fluoride?
The European Academy of Pediatric Dentistry (EAPD) recommends fluoride pastes and gels for everyone - even children and pregnant women - to prevent early stages of tooth decay. Children can begin brushing their teeth with fluoride-containing toothpastes when their first tooth erupts.
Recommended fluoride content in toothpaste:
- up to 4 years
- 200 ppm; - from 4 to 8 years
- 500 ppm; - over 6 years old
and for adults - 1450 ppm.
This recommendation is suitable for anyone living in areas with moderate levels of fluoride in drinking water. Up-to-date information on the fluoride content of water in your region can be found on the website of your local water utility or in our table.
Symptoms, forms and degrees of fluorosis
Dental damage due to excess fluoride is popularly called “strong enamel”, “spotted enamel”. Based on the characteristics of the localization of stains and the degree of fluoride exposure to tooth enamel, several forms of pathology are distinguished:
- Streaked : inconspicuous spots in the form of light stripes, most often on incisors and canines, without destruction of dental tissue.
- Spotted : yellow spots in the center of the crowns of teeth, similar to caries.
- Chalky-mottled : dotted rough spots with specks of yellow, brown color that can be on all teeth.
- Erosive : visually noticeable spots with enamel defects, leading to hypersensitivity and tooth decay.
- Destructive : highly visible areas of tooth decay, corresponding to the last stage of the disease.
Degrees of the disease:
- Easy . Rare milky white streaks or spots are found on only a few teeth. No more than 25% of the enamel is affected.
- Moderate . Spots, specks and streaks are clearly visible on the teeth. The enamel is affected by 50%.
- Average . Brown and yellow inclusions are added to the milky spots. The enamel is significantly thinned, dentin is exposed.
- Heavy . Up to 70% of tooth crowns are affected. The spots are cinnamon and yellow, there are erosive defects, the enamel is chipped, and the teeth are deformed.
Is there an alternative?
With the right approach and judicious use, fluoride is an effective way to treat and prevent tooth sensitivity, caries and enamel demineralization. But, if you still have doubts, there is an alternative - pastes with synthetic hydroxyapatite.
This hydroxyapatite is completely identical to natural hydroxyapatite, the one that makes up tooth enamel. The substance is easily integrated into the crystal lattice of the enamel, strengthening it, reducing sensitivity and preventing the development of caries. The only drawback is that pastes with hydroxyapatite are more expensive than fluoride-containing ones.
Alternative to fluoride
The harm or benefit of fluoride in toothpaste is a largely debatable issue.
Using fluoride toothpaste is possible, and in some cases even recommended. On the other hand, this is not a compulsory measure or a panacea. But the mark on the tube “Fluoride free!” — may well turn out to be a product of marketing. Although there are compounds and components that are an alternative in the prevention of caries and demineralization. Firstly, these are toothpastes with glycerophosphate and calcium hydroxyapatite, which saturate the enamel and resist the effects of acid. Probiotics in toothpastes help maintain optimal oral microflora, and diet and special rinsing solutions will help control pH levels. Natural plant ingredients also help achieve an antibacterial effect. Publisher: Expert magazine about dentistry Startsmile.ru
Author of the material: Yaroslav Ikonnikov
Reverse osmosis plants for purifying fluoride from water
Reverse osmosis plants differ from other types of water treatment equipment in their high productivity and the deepest degree of water purification from fluorine, heavy metal salts, and organic impurities. They are capable of working around the clock, continuously purifying intense water flows in large volumes.
It is beneficial to use the reverse osmosis method to purify water from fluoride. It retains F(-) ions and other impurities, allowing only water molecules to pass through the pores. This ensures output purity of up to 98%. You can set several degrees of purification and separate water flows: for drinking, for household needs.
Osmosis for all industries
To purify large volumes of water, we will assemble a powerful industrial reverse osmosis installation with a line of membrane filters to remove fluoride from water, which operate effectively when the fluoride concentration is exceeded. We will place the necessary elements on a stainless steel frame. We will install shut-off and control equipment, local water supply systems.
We can offer companies equipment of various capacities:
- up to 250 l/hour - for small production,
- up to 500 l/hour - for medium-sized enterprises,
- up to 2000 l/hour - for water supply of especially large industries.
Industrial reverse osmosis units are suitable for any production area that requires clean technical and process fluid. They will allow you to obtain high-quality purification (less than 1 µS/cm), which is very important for microelectronics, for obtaining clean water for laboratories or for preparing injection solutions.
Osmosis for home, for use in households
A membrane filter for water purification from fluoride based on reverse osmosis, installed in a compact household unit, will perfectly cope with everyday tasks. It will fit in a small corner under the sink and provide the whole house with clean water at once.
To reduce the cost of the kit, you can use different methods of purifying water from fluoride. To obtain drinking water, install osmosis with fluoride water filters in the kitchen, and use cartridges that provide less deep cleaning in the bathroom or shower. The external effects of fluorides are not as dangerous as their destructive effect on the body when ingested with drinking water.
Fluorides in pastes
In modern pastes you can most often find:
- Tin fluoride. Inorganic binary compound, tin salt of hydrofluoric acid. SnF2 acts as a reducing agent in many chemical reactions. Prevents the formation of carious cavities and fights gingivitis. Helps with increased tooth sensitivity. Swiss researchers have found that regular use of the substance increases the resistance of enamel to the damaging effects of acids.
- Sodium fluoride. It has the chemical formula NaF. It is highly effective, remineralizes enamel well, and provides caries prevention. It works quite quickly. Suitable for fluoridation of drinking water. By the way, it is precisely this that opponents of fluoride-containing drugs usually speak out against.
- Monosodium phosphate. In pastes it functions as an anti-caries additive. Also serves as a reagent for water fluoridation. Modern manufacturers rarely add Sodium monofluorophosphate to their dental products. This is due to the slow release of beneficial ions from it.
- Aminofluoride. Organic matter. Creates a kind of protective film on the enamel. Quickly and evenly distributed over the enamel. This is the most modern and expensive type of fluoride products.
Fluorides and fluoride for teeth
In its pure form, the component is a gas with a barely noticeable yellow tint. It has a rather strong chemical smell. Compared to other elements of the periodic table, it is very active.
As a result of interaction with other compounds, fluorine is converted into fluorides. There is a huge variety of them. They are used in various fields of medicine and cosmetology. In the 40s, American scientists even saturated water with them to reduce the prevalence of caries in the population. And such experiments turned out to be very successful.
Therefore, it is not surprising that even then fluoride-containing dental rinses and toothpastes could be seen on store shelves. But it is important to understand that they do not contain pure fluoride - they contain fluorides.
Excess fluoride and the appearance of fluorosis
Fluorine is one of the microelements needed by the body. Most of it is found in hard tissues (bone tissue, dentin). The body receives it from food and water. Absorption is slow, in small quantities. We get most fluoride from water (in the form of a fluoride solution). Their concentration should be 1 mg/l (for drinking water). At higher concentrations, hard tissues lose strength, which increases the risk of carious lesions. When there is oversaturation of fluoride, destruction of hard tissues begins and fluorosis appears.