Treatment of cysts with calcium preparations in dentistry
Given the constant and continuous development, modern dentistry successfully combats most diseases of the teeth, gums and the oral cavity itself. One of these problems is a dental cyst.
Tooth cyst
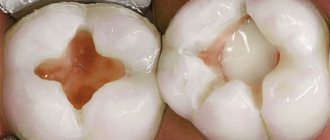
is a disease expressed in the formation of an inflammatory capsule with dense walls, inside of which there is often a mass consisting of bacteria and dead cells. This neoplasm appears in the gums around the root of the tooth, and in the absence of timely and targeted treatment, it can cause undesirable consequences. Over time, the size of the cyst increases, gradually destroying the surrounding bone tissue.
The occurrence of a dental cyst is usually associated with an infection; less often, the cause of the disease can be an injury that can be obtained in a completely trivial way - for example, cracking nuts with your teeth.
To determine the presence of a dental cyst, an X-ray examination of the affected area of the jaw is performed. Most often, the patient does not even suspect the presence and development of a cyst, since it passes painlessly
without causing any discomfort. Only during exacerbation does profuse tissue inflammation occur, accompanied by purulent formations and “flux.”
For successful treatment of the disease, its timely diagnosis is very important. Modern dentistry uses two methods to treat inflammation: surgical and therapeutic.
For therapeutic treatment of cysts, the inflamed tooth is drilled and the root canal is disinfected using a special solution. However, the result of these manipulations is not always successful, therefore, six months after the treatment, a re-examination with X-rays will be required to exclude re-inflammation.
Treatment of cysts with a more modern therapeutic method used in dentistry is the use of depophoresis
.
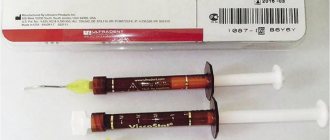
During the treatment, calcium is used, or rather copper-calcium hydroxide, which is injected into the canal of the diseased tooth. At the same time, the tooth is exposed to a weak electric current - for better penetration of the suspension into the cyst and its removal. After three sessions of depophoresis, the tooth is filled. Also, in the treatment of dental cysts, preparations based on calcium and iodine are used ( Metapex
,
Calasept,
etc.).
When using the surgical method in dentistry, the cyst is removed along with the damaged part of the tooth root (cystectomy), or the entire root and part of the tooth above it are removed (hemisection), subsequently placing a crown on it. The site of the resulting defect in the bone tissue is filled with bone material.
Complications in endodontics
Author: Zuhair Alkhatib
Translation: Roman Ragozin
Calcium hydroxide
Calcium hydroxide [Ca(OH)2] has been used in dentistry for many years. In root canal treatment, its use as a temporary attachment has a positive effect on the healing of periapical inflammation. Due to this property, calcium hydroxide has become a widely used preparation for temporary intracanal placement. Calcium hydroxide is an effective antibacterial agent, mainly due to the high acidity of the environment (pH) and its destructive effect on bacterial cells and protein structures. It has been established that when calcium hydroxide is applied in cases of extensive periapical lesions, conditions favorable for healing are created and osteogenesis is stimulated. Calcium hydroxide is a shapeless, fine, granular powder with strong base properties and a density of 2.1. Slightly soluble in water and insoluble in alcohol. Due to the lack of radiopacity of calcium hydroxide, it is difficult to determine its presence on radiographs. This is the main reason for adding various radiopaque materials (barium sulfate [BaSO4], bismuth, and other compounds containing iodine and bromine) to the paste, thereby allowing the detection of lateral and accessory canals, resorptive defects, fractures and other structures. At the same time, in cases of uncovered root apex, it is recommended to use calcium hydroxide without radiopaque impurities, since the degree of leaching of calcium hydroxide is assessed by its relative radiodensity in the canal in subsequent visits.
Etiological factors
During root canal treatment, temporary material may inadvertently be removed beyond the root apex, which may result in complications such as hypoesthesia and paresthesia of the inferior alveolar nerve. As a rule, such complications are observed when the material is removed under high pressure, or when there is a neurotoxic effect on the neurovascular bundle. The released agent causes changes in the surrounding bone and also affects the inferior alveolar nerve. Extrusion is also observed with a wide apical foramen in immature permanent teeth. Complications can arise when a syringe with calcium hydroxide is immersed to a depth sufficient to create pressure above arterial pressure, causing the paste to penetrate the bloodstream. It should be noted that calcium hydroxide particles are small enough to penetrate the capillaries, which can cause mechanical obstruction, or induce crystallization in the bloodstream, blocking circulation. Conclusion
Depophoresis
Depophoresis is the newest method of treating teeth with difficult to pass root canals. Increases the reliability of canal filling and the effectiveness of treatment of periodontitis, cystogranulomas, teeth with fragments of instruments.
This method, which belongs to the group of “high technologies” in dentistry, is widely used in our clinics for endodontic treatment of root canals.
The main goal of endodontic treatment is to ensure the permanent sterility of the remaining tooth in the jaw. This cannot be achieved using traditional approaches in endodontics, since disinfectants are not able to reach the entire complex system of root microcanals through natural diffusion from the main root canal. There are also teeth for which access to the root canals is very difficult due to anatomical features (for example, the bend of the root canal at an angle of more than 60 degrees). Therefore, classical endodontic treatment does not guarantee complete success due to the unpredictability of microbiological processes in the complex root canal system. The consequences of classical endodontic treatment strongly depend on the degree of infection of the root dentin and the immunological status of the patient.
In recent years, a fundamentally different method of endodontic treatment of a complex root canal system has been proposed - copper-calcium hydroxide depophoresis . During treatment under the influence of an electric field, from the area of high content (depot) of the copper-calcium hydroxide suspension (in the main root canal), forced diffusion of hydroxide ions (having a strong bactericidal effect) and hydroxycuprate ions occurs into the entire canal system, including side microchannels, microholes . This leads to complex chemical and biochemical reactions and processes involving biological tissues and microorganisms. After 2-3 sessions of depophoresis (5-7 minutes for each canal), the canal is filled with a special material - atatamyte.
As a result, the tooth root becomes sterile, completely protected from the penetration of microorganisms and can serve as a support for further restoration and prosthetics. The method is also used in the treatment of chronic periodontitis. According to numerous data, the depophoresis method leads to positive results in 92% of cases and is therefore often the only alternative to tooth extraction.
A shining Hollywood smile from leading specialists in aesthetic dentistry. Make an appointment!
Reasons and purposes of application
The scope of application of the substance in dentistry is quite narrow. The direct purpose of calcium hydroxide in endodontics is the treatment and filling of root canals. The basis for use is the diagnosis of pathological processes in the area of the pulp and hard tissues of the tooth.
When the material remains in the channel cavity for more than two weeks, cementation of the powder occurs, which leads to complete sealing of the channel. In certain cases, this effect is used purposefully by dentists if other treatment methods are unacceptable.
It should be clarified that preparations with calcium hydroxide can be used in the surgical treatment of damage to the jaw structure, as well as in bone grafting.
Calcium hydroxide is also used in the dental field for intra-root therapeutic effects. Calcium-based materials are used if the following diseases are diagnosed:
Treatment with calcium hydroxyl cements is relevant for periodontitis
- pulpitis;
- inflammatory processes in bone tissue;
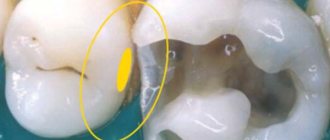
- neoplasms (granuloma, cyst);
- periodontitis;
- periodontitis;
- deep caries.
Calcium hydroxide can also be used after surgery to remove tumors or nerves. Used to fill canals before installing permanent fillings or implants.
Unlike many other medications used in dentistry, calcium hydroxide does not have side effects and can be contraindicated only if you are intolerant to the chemical itself.
Calcium hydroxide: yesterday, today, tomorrow
Authors:
1st Department of Therapeutic Dentistry BSMU L.A. Kazeko, I.N. Fedorov Calcium hydroxide: yesterday, today, tomorrow
Calcium hydroxide Ca(OH)2 is a strong base, slightly soluble in water. A saturated solution of calcium hydroxide is called lime water and is alkaline. In air, limewater quickly becomes cloudy due to the absorption of carbon dioxide and the formation of insoluble calcium carbonate [3].
Calcium hydroxide (“slaked lime”) is a white, very fine powder, slightly soluble in water (1.19 g/l), solubility can be increased by glycerin and sucrose. Hydrogen index (pH) is about 12.5. Calcium hydroxide is very sensitive to contact with atmospheric carbon dioxide, which transforms it into calcium carbonate. The drug should be stored in sealed packaging away from light; it can be stored in a supersaturated aqueous solution (distilled water) in an airtight bottle.
The basis for the use of calcium hydroxide in endodontics was information about the etiology and pathogenesis of pulpitis and apical periodontitis. The most common cause of these diseases is microorganisms in the root canal system of the tooth. Kakehashi et al. (1965), Moller et al. (1981) showed in experiments that periapical inflammation and destructive processes around the apex of the tooth develop only with the participation of root canal microorganisms [2, 5, 15]. Favorable factors for the existence of microflora are the complex anatomy of the root canals, the ability of bacteria to penetrate into the dentinal tubules to a depth of 300 microns, anaerobic development conditions, the ability to feed from living or necrotic pulp, salivary proteins, and periodontal tissue fluid. Thus, the quality of endodontic treatment is determined by the quality of disinfection of the root canal system [6, 11].
Endodontic instrument breakage, root perforation, ledges, and overfilling or underfilling are considered to be the main causes of endodontic failure. However, in most cases, these errors do not affect the outcome of endodontic treatment until a concomitant infection occurs. Of course, gross errors prevent or make it impossible to complete intracanal procedures, but the chances of successful treatment increase significantly if the infectious and toxic contents of the root canals are effectively removed before filling.
Microorganisms that survive instrumentation and irrigation quickly multiply and repopulate root canals that remain empty between visits [1, 5]. The likelihood of reinfection depends on the quality of root canal filling and the usefulness of the coronal restoration. However, in all cases where bacteria remain in the root canal system, there is a risk of further development of periapical changes [4, 18].
In untreated teeth with primary intracanal infection, one or more species of bacteria are usually present, with no apparent predominance of facultative or anaerobic forms. In case of secondary infection if treatment fails, a mixed infection is present, with gram-negative anaerobic strains dominating [1, 4].
There are differing opinions regarding the necessary number of treatment steps for patients with periapical problems. Thus, some authors justify the need to treat infected root canals in several visits, using temporary intracanal dressings, which allows for the gradual and controlled destruction of microorganisms in them. Others suggest preventing the growth of remaining microorganisms by depriving them of nutrition and living space by fully debridement, disinfection, and 3D filling of the root canals during the first and only visit.
Anti-inflammatory and antibacterial activity of calcium hydroxide
Instrumental treatment of the root canal reduces the number of microorganisms by 100–1000 times, but their complete absence is observed only in 20–30% of cases. Antibacterial irrigation with 0.5% sodium hypochlorite solution increases this effect to 40–60% [2]. It is very difficult in practice to achieve complete disinfection of infected root canals even after complete mechanical cleaning and irrigation with antiseptic solutions. It is possible to destroy bacteria remaining in the root canal by temporarily filling the root canal with antimicrobial agents until the next visit. Such drugs should have a wide spectrum of antibacterial action, be non-toxic and have physicochemical properties that allow them to diffuse through the dentinal tubules and lateral canals of the tooth root system [6, 8].
Calcium hydroxide is widely used as a temporary intracanal agent in endodontics, which decomposes into calcium ions and hydroxide ions in an aqueous solution. The main biological properties of hydroxide: bactericidal activity, anti-inflammatory properties, tissue solubility, hemostatic effect, inhibition of tooth tissue resorption, stimulation of bone regeneration processes [2, 4, 19].
Calcium hydroxide has bactericidal activity due to its high alkalinity and the release of hydroxide ions - highly active free radicals - in the aqueous environment. Their effect on bacterial cells is explained by the following mechanisms:
– damage to the cytoplasmic membrane of the bacterial cell, which plays an important role in the preservation of the cell. It is the cell membrane that provides selective permeability and transport of substances, oxidative phosphorylation in aerobic strains, production of enzymes and transport of molecules for the biosynthesis of DNA, cellular polymers and membrane lipids. Hydroxide ions from calcium hydroxide cause lipid oxidation, which leads to the formation of free lipid radicals and the destruction of phospholipids, which are structural components of cell membranes. Lipid radicals initiate a chain reaction, as a result of which unsaturated fatty acids are lost and cell membranes are damaged;
– denaturation of proteins due to the fact that the alkaline environment of calcium hydroxide causes the destruction of ionic bonds that provide the structure of proteins. In an alkaline environment, the polypeptide chains of enzymes are chaotically combined and transformed into disordered formations. These changes often lead to loss of biological enzyme activity and disruption of cellular metabolism;
– damage to microbial DNA, with which hydroxide ions react, causing its cleavage and leading to damage to genes due to disruption of DNA replication. In addition, free radicals can independently cause destructive mutations.
The bactericidal effect of calcium hydroxide depends on the concentration of hydroxide ions, which is high only in the area of direct contact with the drug. When calcium hydroxide diffuses deeper into dentin, the concentration of hydroxide ions decreases due to the action of buffer systems (bicarbonate or phosphate), acids, proteins and CO2, and the antibacterial activity of the drug may decrease or slow down [1]. Neutralization of high pH calcium hydroxide can also occur as a result of coronal microleakage, leakage of tissue fluid through the root apex, the presence of necrotic masses in the canal, and the production of acidic substances by microbes. In the root canal the pH is 12–12.5, in the adjacent dentin, where there is close contact with the hydroxide, the pH varies from 8 to 11, and in the depths of the dentin the pH values are 7–9. The highest pH values were obtained in the period from 7 to 14 days after adding an aqueous suspension of calcium hydroxide to the channel [1, 2].
Microorganisms differ in their resistance to changes in pH; most of them reproduce at pH 6–9. Some strains can survive at pH 8–9 and are usually the cause of secondary infection. Enterococci (E. faecalis), resistant to pH 9–11, are not normally found in root canals or are present in small quantities in untreated teeth. They play an important role in unsuccessful endodontic treatment and are often (32–38% of cases) present in teeth with apical periodontitis.
One of the important components of the effective disinfectant effect of the drug in endodontics is its ability to dissolve and penetrate into the root canal system. Alkalis (NaOH and KOH) are highly soluble and can diffuse deeper than calcium hydroxide. These substances have pronounced antibacterial activity. But high solubility and active diffusion enhance the cytotoxic effect on the cells of the body. Due to their high cytotoxicity, they are not used in endodontics. Calcium hydroxide is biocompatible, since due to its low water solubility and diffusion, a slow increase in pH occurs, which is necessary to destroy bacteria localized in dentinal tubules and other hard-to-reach anatomical formations. Because of these features, calcium hydroxide is an effective but slow-acting antiseptic [1, 5, 19].
The time required for optimal root canal disinfection with calcium hydroxide has not yet been precisely determined. Clinical studies provide conflicting results. Cwikla et al. (1998) found that in 90% of cases there was no bacterial growth after 3 months of hydroxide use [13]. In a study by Bystrom et al. (1999) calcium hydroxide effectively killed microorganisms within 4 weeks of use. Reit and Dahlen used the drug for 2 weeks - infection persisted in 26% of root canals [2]. In an experiment by Basrani et al. After one week of using calcium hydroxide, bacteria remained in the canals in 27% of cases [12].
Mechanisms of resistance of microorganisms to the action of intracanal disinfectants
Factors that determine the resistance of microorganisms to the action of disinfectants and the ability to survive after the use of intracanal (temporary and permanent) filling materials:
— neutralization of the drug by buffer systems or products of bacterial cells;
— exposure of the disinfectant in the root canal is insufficient to destroy microorganisms;
— low antibacterial effectiveness of the drug against root canal microorganisms;
— the effect of the drug on microorganisms is limited for anatomical reasons;
— the ability of microorganisms to change their properties (genes) after environmental changes [1, 4].
An important mechanism of bacterial resistance is their existence in the form of a biofilm. Biofilm is a microbiological population (bacterial ecosystem) associated with an organic or inorganic substrate, surrounded by bacterial waste products. Various strains of microorganisms collected in a biofilm are capable of organizing associations for joint survival and have increased resistance to antimicrobial agents and protective mechanisms [10]. Over 95% of naturally occurring bacteria are found in biofilms.
It is more difficult to destroy bacteria in biofilms than in planktonic suspensions unless the disinfectant has the ability to dissolve tissue. When re-treating infected teeth, calcium hydroxide cannot 100% kill persistent bacteria (E. faecalis), which is able to multiply between dental visits. Of great importance is a complete preparation and cleansing of the canal from all microorganisms on the first visit (using copious rinses with sodium hypochlorite). Prevention of re-infection of the root canal is achieved by completely sealing the tooth crown using high-quality temporary fillings [4].
Effect of solvents on the antibacterial activity of calcium hydroxide
Substances used as a medium for calcium hydroxide have different water solubility. An optimal environment should not change the pH of calcium hydroxide. Many solvents do not have antibacterial activity, such as distilled water, saline, and glycerin. Phenol derivatives, such as paramonochlorophenol, camphor phenol, have pronounced antibacterial properties and can be used as a hydroxide medium. Calcium hydroxide with paramonochlorophenol has a long range of action and destroys bacteria in areas remote from the places where the paste is applied [5].
Siqueira et al. found that calcium hydroxide in saline does not kill E. faecalis and F. nucleatum in dentinal tubules within a week of use. And calcium hydroxide paste with paramonochlorophenol and glycerol effectively killed bacteria in the tubules, including E. faecalis, within 24 hours of use. That is, paramonochlorophenol enhances the antibacterial activity of calcium hydroxide [20].
The results of a study on the disinfection of dentinal tubules using three preparations of calcium hydroxide (Ca(OH)2 in distilled water, Ca(OH)2 with potassium iodide and Ca(OH)2 with iodoform (Metapex)) showed that Ca(OH)2 in in its pure form it is less effective in killing microbes in dentinal tubules. In channels with calcium hydroxide, growth of some microorganisms (E. faecalis, C. albicans) to a depth of 250 µm was observed for 7 days. This is explained by the fact that Ca(OH)2 has a low degree of permeability and its high pH (12) is partially neutralized by dentin buffer systems. Ca(OH)2 with potassium iodide is more effective than pure hydroxide. But Metapex paste (Ca(OH)2 with iodoform) turned out to be the most effective: in addition to E. faecalis, it neutralized other microbes and penetrated into the tubules to a depth of more than 300 μm (Cwikla et al.) [1, 13].
Abdullah et al. (2005) studied the effectiveness of various intracanal agents (calcium hydroxide, 0.2% chlorhexidine, 17% EDTA, 10% povidone-iodine, 3% sodium hypochlorite) against E. faecalis strains contained in bacterial biofilms. As part of the biofilm, E. faecalis was killed in 100% of cases by 3% sodium hypochlorite after 2 minutes and 10% povidone-iodine after 30 minutes. Calcium hydroxide partially eliminated these bacteria [9].
Since some microorganisms, especially E. faecalis, are resistant to calcium hydroxide, its combination with other antimicrobial agents that increase its activity, for example, idoform, camphor paramonochlorophenol, is justified. Having low surface tension, fat-soluble phenols penetrate deep into tooth tissue.
In endodontics, chlorhexidine, which is effective against many bacteria that cause endodontic infection, is recommended for widespread use as an irrigant and intracanal dressing. The chlorhexidine molecule, interacting with the phosphate groups of the bacterial cell wall, penetrates the bacterium and has an intracellular toxic effect [12, 19].
Calcium hydroxide in combination with 2% chlorhexidine gel has increased antimicrobial activity, especially against resistant microorganisms. Chlorhexidine in gel form has such positive properties as low toxicity for periodontal tissues, viscosity, which allows the active substances to be kept in constant contact with the walls of the root canal and dentinal tubules, and water solubility. The combination of chlorhexidine gel and calcium hydroxide against E. faecalis in infected root dentin has been found to be highly effective [15]. High pH (12.8) in the first two days increases the penetration ability of the drugs.
Effective against E. faecalis after 1, 2, 7 and 15 days of application of 2% chlorhexidine gel. According to Gomes et al., 2% chlorhexidine gel has greater antibacterial activity against E. faecalis than calcium hydroxide, but this ability is lost when used for a long time. This is confirmed by other studies, even when using chlorhexidine in the form of a solution or gel at concentrations of 0.05%, 0.2% and 0.5%. The combination of chlorhexidine and calcium hydroxide inhibits the growth of E. faecalis by 100% after 1-2 days of contact [12, 15].
Calcium hydroxide as a physical barrier
Secondary intracanal infections are caused by microorganisms that enter the canal during treatment, between visits or after dental treatment. The main sources of secondary infection: dental plaque on teeth, caries, infected endodontic instruments. Causes of infection between visits may include microleakage through a temporary filling due to its destruction; tooth fracture; delay in replacing a temporary filling with a permanent one, when the tooth remains open for drainage. Secondary infection allows the emergence of new, virulent microorganisms that cause acute periapical inflammation [4, 7].
Intracanal preparations destroy bacteria remaining after chemomechanical treatment of the canal, and are also used as a physical and chemical barrier that prevents the proliferation of microorganisms and reduces the risk of reinfection from the oral cavity. Reinfection of the canal is possible due to the fact that the drug is dissolved by saliva, saliva seeps into the space between the medication and the walls of the canal. However, if a drug has an antibacterial effect, it will first be neutralized and only then will bacterial invasion occur.
To prevent reinfection, the sealing ability of calcium hydroxide is more important than its chemical activity, since it has low water solubility, dissolves slowly in saliva, remains in the canal for a long time, delaying the movement of bacteria towards the apex [4, 5]. Despite the use of solvents, calcium hydroxide acts as an effective physical barrier, destroying some of the remaining bacteria and preventing their growth, limiting the space for reproduction [1, 18].
As a reliable isolating barrier for various endodontic problems (perforation of the cavity bottom, tooth root, root resorption, etc.), a new class of materials - mineral trioxide aggregate (ProRoot MTA) - has been proposed. The basis of MTA is calcium compounds [4, 7].
The influence of calcium hydroxide on the quality of permanent root canal filling
Before permanent obturation, calcium hydroxide is removed from the root canal using sodium hypochlorite, saline and endodontic instruments.
Lambrianidis et al. (1999) investigated the possibility of removing certain calcium hydroxide preparations from root canals: Calxyl (42% calcium hydroxide) and an aqueous suspension (95% calcium hydroxide). The percentage of calcium hydroxide did not affect the effectiveness of cleaning the root canal walls. Paste residues can affect the mechanical properties of the sealer and impair the apical seal. There is an opinion that it is impossible to completely remove the paste from the walls of the root canal [17].
Residual calcium hydroxide negatively affects the hardening of zinc oxide eugenol sealers, as it interacts with the eugenol of the paste to form calcium eugenolate. In the clinic, this may manifest itself as blocking the advancement of the gutta-percha pin along the entire working length of the canal. If calcium hydroxide residues are not completely removed, they become compacted apically or in the recesses of the canal, which mechanically interferes with effective canal filling, complicates apical sealing and can affect the outcome of endodontic treatment. It is preferable to remove the apical calcium hydroxide plug.
Calcium hydroxide is effectively removed from the canal walls with hand instruments and rinsing with sodium hypochlorite and 17% EDTA [5, 13]. Difficulties in cleaning root canals after temporary filling are caused by paste-forming substances and fillers, and not calcium hydroxide. Water-based calcium hydroxide preparations (especially those prepared ex tempore) are absolutely free from these disadvantages. Moreover, calcium hydroxide-based sealers should be considered the materials of choice for permanent obturation of root canals after their temporary filling with calcium hydroxide.
Indications for temporary filling of root canals
The use of non-hardening pastes based on calcium hydroxide is indicated as a temporary intracanal agent for the treatment of acute forms of apical periodontitis, destructive forms of chronic apical periodontitis, cystogranulomas, radicular cysts, progressive root resorption, teeth with an unformed root apex in pediatric practice.
Method of using calcium hydroxide:
1) calcium hydroxide in powder form is mixed to a paste with distilled water or glycerin;
2) the paste is injected into a carefully instrumentally and medicinally treated root canal using a canal filler;
3) to ensure adherence to the root dentin, the paste is compacted with a paper pin and covered with an airtight bandage.
Features of the use of calcium hydroxide for different conditions of the apical periodontium. In acute forms of apical periodontitis, temporary filling with calcium hydroxide is intended to have an anti-inflammatory and antimicrobial effect. Calcium hydroxide is introduced into the root canal loosely, without compaction, first for a day, then again for 1–3–7 days, depending on the clinical picture. In case of acute periapical abscess, periostotomy is performed according to indications.
In case of chronic destructive processes in the apical periodontium, the goal is to provide not only an anti-inflammatory and antimicrobial effect, but also to stimulate reparative processes in the bone. Calcium hydroxide is injected into the root canal with compaction to the walls for 3–8 weeks; the time of renewal of the material depends on the clinical picture. Treatment is designed for a period of 0.5 to 1 year, its duration depends on the degree of infection of the root canal, the body's resistance, the patient's age, and motivation to cooperate. Restoration of the zone of destruction of the apical periodontium continues after constant filling of the root canal with a calcium hydroxide-based sealer for 3–5 years.
Filling teeth with apical periodontitis at the first visit does not eliminate acute inflammation. Resorption of cement and dentin persists even 9 months after filling. Moreover, in 80% of cases a chronic process is formed. If, after drainage, the canal was filled with calcium hydroxide for 7 days before obturation, the periapical defect was replaced with new bone tissue, although in 18.8% of cases the inflammation progressed [2, 4].
Acute reactions during hermetic closure of the coronal cavity persisted in only 5% of teeth in the presence of a periapical abscess. A temporary dressing and hermetic filling prevent re-infection of the canal and increase the success of conservative treatment to 61.1% (compared to 22.2% without an antibacterial dressing) [5, 18].
When calcium hydroxide is used as a temporary dressing, after 3 years complete bone regeneration is observed in 82% of periapical lesions, even large ones. In 18% of cases, bone defects remained or slightly decreased in size. The most active reduction in the size of the defect was observed in the first year of treatment. The first positive signs were detected on radiographs 12 weeks after the introduction of the Ca(OH)2 dressing, and on digital radiographs after 3–6 weeks [2].
"Yesterday" calcium hydroxide. Information materials, scientific articles about calcium hydroxide preparations 20-30 years ago convinced (and convinced) us of its unique abilities: pastes based on calcium hydroxide have a highly alkaline reaction, unlimited bactericidal action, and the ability to stimulate reparative processes in bone tissue.
The use of calcium hydroxide in endodontics has expanded the indications for conservative treatment of destructive processes in the apical periodontium. It is now possible to fully preserve teeth that were previously considered hopeless. “The biocompatibility of calcium hydroxide has made it a polyvalent drug adapted to almost all clinical situations encountered in endodontics” [2]. Recommendations have appeared on the mandatory stage of temporary filling of root canals during endodontic treatment: “This is useful!”
“Today,” a baggage of clinical observations has been accumulated that confirm the very high effectiveness of calcium hydroxide (Fig. 1–4; from the authors’ own observations). High-quality implementation of all stages of endodontic treatment in combination with temporary filling of root canals with calcium hydroxide allows us to recognize this method of treatment as organ-saving.
But today in the dental literature the issues of the breadth of the antibacterial action of calcium hydroxide preparations, targeted effects on the most resistant and aggressive strains of microorganisms that cause the development of periapical foci of destruction, re-infection and the development of exacerbations are discussed.
So, A.A. Antanyan writes [1]: “A multilateral analysis of the scientific literature of recent years (2003–2006) has shown that calcium hydroxide has many disadvantages that call into question its routine and mass use in endodontics. In modern endodontics, the most important is complete preparation, cleansing the canal from infection on the first visit (using copious rinses with sodium hypochlorite) and preventing re-infection of the canal by fully sealing the tooth crown using high-quality temporary fillings. Therefore, in many clinical situations, additional disinfection with calcium hydroxide is not necessary.”
"Tomorrow" calcium hydroxide. Experience in the clinical use of calcium hydroxide shows that the need for its use in endodontics cannot be justified solely by its antimicrobial effectiveness, which in past years was primarily responsible for the treatment outcome. With the advent of sensitive methods of microbiological research, with the expansion of the range of highly effective means for irrigation of root canals, the possibilities and properties of calcium hydroxide as a material for temporary filling can be rethought and reevaluated. But not discounted! In difficult clinical situations involving endodontic treatment and re-treatment of teeth, thanks to calcium hydroxide preparations, it is possible to preserve the patient’s teeth and health.
LITERATURE
1. Antanyan A. A. // Endodontics today. – 2007. – No. 1. – P. 59–69.
2. Beer R., Bauman M.A. Illustrated guide to endodontology. – M., 2006. – 240 p.
3. Glinka N.L. General chemistry: Textbook. manual for universities. – 20th ed., rev. / Ed. Rabinovich V.A. – L., 1979. – P. 614–617.
4. Gutman J.L., Dumsha T.S., Lovdel P.E. Solving problems in endodontics: Prevention, diagnosis and treatment / Transl. from English – M., 2008. – 592 p.
5. Poltavsky V.P. Intracanal medicine: Modern methods. – M., 2007. – 88 p.
6. Simakova T.G., Pozharitskaya M.M., Sinitsyna V.I. // Endodontics today. – 2007. – No. 2. – P. 27–31.
7. Solovyova A.B. // Dentsplay News. – 2003. – No. 8. – P. 14–16.
8. Kholina M.A. // Dentplay News. – 2007. – No. 14. – pp. 42–45.
9. Abdullah M., Yuan-Ling N., Moles D., Spratt D. // J. Endod. – 2005. – V. 31, N 1. – P. 30-36.
10. Allais G. // New in dentistry. – 2005. – No. 1. – P. 5–15.
11. Athanassiadis B., Abbott PV, Walsh LJ // Austr. Dent. J. – 2007. – Mar; 52 (Suppl 1). – S. 64–82.
12. Basrani B., Santos JM, Tjäderhane L. et al. // Oral Surg. Oral Med. Oral Pathol. Oral radiol. Endod. – 2002. – Aug; 94(2). – P. 240–245.
13. Cwikla S., Belanger M., Giguere S., Vertucci F. // J. Endod. – 2005. – V. 31, N 1. – P. 50-52.
14. Ercan E., Ozekinci T., Atakul F., Gül K. // J. Endod. – 2004. – Feb; 30(2). – P. 84–87.
15. Gomes B., Souza S., Ferraz C. // Intern. Endod. J. – 2003 – V. 36. – P. 267–275.
16. Heckendorff M., Hulsmann M. // New in dentistry. – 2003. – No. 5. – P. 38–41.
17. Lambrianidis T., Margelos J., Beites P. // Intern. Endod. J. – 1999. – V. 25, N 2. – P. 85–88.
18. Regan JD, Fleury AA // J. Ir. Dent. Assoc. – 2006. – Autumn; 52 (2) – P. 84–92.
19. Sathorn C., Parashos P., Messer H. // Intern. Endod. J. – 2007. – V. 40, Issue 1. – P. 2–10.
20. Siqueira JF, Paiva SS, Rôças IN // J. Endod. – 2007. – May; 33(5). – P. 541–547.
Modern dentistry. – 2009. – No. 2. – P. 4-9.
Mechanism of action
As a result of applying dissolved powder to the root canals, a reaction begins to occur between the substance, tissue cells and the microflora of the inside of the tooth. The hydroxide diffuses through the dentin channels and penetrates deep into the pulp.
The mechanism of action of calcium hydroxide is designed to provide an antimicrobial, anesthetic and therapeutic effect. Due to the long-term presence of the drug in the tooth cavity, the treated area is completely disinfected and loses sensitivity, which allows further treatment and the installation of fillings or therapeutic pads without any risks.
In addition to the main antiseptic effect, some preparations based on calcium hydroxide are capable of destroying certain types of organic tissue, which makes it possible to “kill” the nerve fibers in the canals (devitalize the pulp), thus achieving the effect of “insensitivity” of the tooth.
Application for temporary filling
The main area of application of preparations based on hydroxide and pure calcium hydroxide powder is the tooth filling procedure. Materials with a chemical are used in the form of temporary and permanent fillings.
Since drugs change the microflora in the area of their application, and can also harden, their use must be carried out according to a certain scheme.
During the procedure for installing a temporary filling, a specialist must perform the following actions:
- measure the length of the processed channel;
- mix calcium powder with saline solution (use a ready-made preparation);
- dip the desired channel filler into the material;
- insert the instrument into the canal, 1 mm short of its end;
- start the rotation of the channel filler to process the desired areas.
Only after the process has been repeated three to four times can the dentist seal the tooth with a small layer of cement.
To increase the effectiveness of treatment, you should adhere to the following rules:
- the root canal should be opened as much as possible;
- after dilution, the finished material should have a creamy consistency;
- the channels must first be cleaned and treated;
- the residence time of the material in the tooth cavity is not less than 2, but not more than 4 weeks.
How to properly mix cement based on calcium hydroxide: