Eugenol
| Names | |
| Preferred IUPAC name 2-methoxy-4-(prop-2-en-1-yl)phenol | |
Other names
| |
| Identifiers | |
| Number of CAS |
|
| 3D model (JSmol) |
|
| CHEBY |
|
| CHAMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.002.355 |
| IUPHAR/BPS |
|
| KEGG |
|
| PubChem C.I.D. |
|
| UNII |
|
| CompTox Control Panel (EPA) |
|
InCHI
| |
Smiles
| |
| Characteristics | |
| Chemical formula | C 10 H 12 O 2 |
| Molar mass | 164.204 g mol -1 |
| Density | 1.06 g/cm3 |
| Melting temperature | -7.5 °C (18.5 °F, 265.6 K) |
| Boiling point | 254 °C (489 °F, 527 K) |
| Acidity ( pKa ) | 10.19 at 25 °C |
| Magnetic susceptibility (χ) | −1.021 × 10 −4 cm 3 / mol |
| Viscosity |
|
| Dangers | |
| NFPA 704 (fire diamond) | 2 1 0 |
| Flash point | 104 °C (219 °F, 377 K) |
| Related compounds | |
| Related compounds | 2-phenethyl propionate |
| Unless otherwise stated, data is for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| N check (what is there?)YN | |
| Links to infoboxes | |
Eugenol
/j¯udʒɪnɒl/ is an allyl chain substituted by guaiacol, a member of the allylbenzene class of chemical compounds.
[2] It is a colorless to pale yellow aromatic oily liquid extracted from certain essential oils, especially clove oil, nutmeg, cinnamon, basil, and bay leaf. [3] [4] [5] [6] It is present in concentrations of 80–90% in clove bud oil and 82–88% in clove leaf oil. [7] Eugenol has a pleasant, spicy clove scent. [8] The name comes from Eugenia caryophyllata
, a former Linnaean term for carnation.
(The currently accepted name is Syzygium aromaticum
.[9])
Modern use[edit]
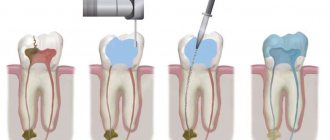
Eugenol is used in perfumes, fragrances and essential oils. It is also used as a local antiseptic and anesthetic. [10][11] Eugenol can be combined with zinc oxide to form eugenol zinc oxide, which is used for restoration and prosthetics in dentistry. For people with dry socket as a complication of tooth extraction, packing the dry socket with a zinc oxide eugenol paste on iodoform gauze is effective in reducing acute pain. [12] Eugenol oxide and zinc paste are also used for filling root canals. [13]
It is one of many compounds attractive to males of various species of orchid bees, which appear to collect chemicals to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study. [14] It also attracts female cucumber beetles. [15] Recently, it was discovered that eugenol and isoeugenol, volatile aromatic compounds of flowers, are catalyzed by a single type of enzyme from the genus Gymnadenia,
and the gene encoding this enzyme is the first functionally characterized gene in these species. [16]
Clove oil is becoming increasingly popular as an anesthetic for use on aquarium fish, as well as on wild fish for sampling for research and management purposes. [17] [18] Where available, it provides a humane method of euthanizing sick and diseased fish, either by direct overdose or by euthanasia before overdosing on eugenol. [19]
Drug interactions
Gevkamen is slightly absorbed by the body, so the likelihood of its interactions with drugs from other groups is extremely low.
In any case, you need to remember about the terpene substances that are part of this medicine. They stimulate liver microsomal enzymes, which can alter the metabolism of certain medications. Combined use of drugs from other groups with Gevkamen may reduce their effectiveness.
The simultaneous use of this ointment with nonsteroidal anti-inflammatory drugs with local application increases the beneficial effects of the latter, deepens and accelerates their entry into soft tissues.
Simultaneous application of dimethyl sulfoxide with Gevkamen can increase the absorption of its constituent components (for example, essential oils), increase the depth and effectiveness of their therapeutic effects.
Biosynthesis [edit]
The biosynthesis of eugenol begins with the amino acid tyrosine. L-tyrosine is converted to p-coumaric acid by the enzyme tyrosine ammonia lyase (TAL). [20] Hence, p
-coumaric acid is converted to caffeic acid by
p
-coumarate 3-hydroxylase using oxygen and NADPH.
S-adenosylmethionine (SAM) is then used to methylate caffeic acid to form ferulic acid, which in turn is converted to feruloyl-CoA by the enzyme 4-hydroxycinnamoyl-CoA ligase (4CL). [21] Feruloyl-CoA is then reduced to coniferaldehyde by cinnamoyl-CoA reductase (CCR). Coniferaldehyde is then reduced to coniferyl alcohol by cinnamyl alcohol dehydrogenase (CAD) or sinapyl alcohol dehydrogenase (SAD). Coniferyl alcohol is then converted to an ester in the presence of the substrate CH3COSCoA, forming coniferyl acetate. Finally, coniferyl acetate is converted to eugenol by the enzyme eugenol synthase 1 and the use of NADPH. [ citation needed
]
Biosynthesis of eugenol
Properties
Eugenol is a colorless liquid that turns yellow in air with a strong clove odor. Soluble in propylene glycol and essential oils, soluble in 50% ethanol in a ratio of 1:5÷1:6, insoluble in water.
- Mm. 164.2
- Boiling point = 252.7°C
- d422=1.0664
- nD20=1.5410
- TVsp = 110°C
- Threshold odor concentration 2.38·10−8 g/l
- LD50 2.68 g/kg (for rats, orally).
When interacting with aqueous solutions of alkalis, it forms soluble salts (eugenolates). Upon oxidation, it forms vanillin. When heated with alkalis, with platinum on carbon it isomerizes to isoeugenol ( formula II
).
Pharmacology [edit]
Eugenol and thymol have been found to have general analgesic properties. Like many other anesthetics, these 2-alkyl(hydroxy)phenols have been found to act as positive allosteric modulators of the GABA A receptor. Although eugenol and thymol are too toxic and not effective enough for clinical use, these results led to the development of 2- substituted phenolic anesthetics, including propanidide (which was later withdrawn) and the widely used propofol. [22] Eugenol, which has a similar structure to myristicin, has the general property of inhibiting MAO-A and, to a lesser extent, MAO-B in humans. [23]
Here are collected articles, abstracts, textbooks on various areas of chemistry. All works are checked by the administrator before publication. If you have interesting information that you are ready to share with Internet users, send it to us and we will definitely publish it.| List of medicines presented in Russia. Contains information on 15,767 medicines, including synonyms of names. | ||
| Auto-equalizer - selection of chemical reaction coefficients | www.webqc.org |
| Online equalizer of chemical reactions. Selects the coefficients of a given reaction. Works with classical molecular reactions, redox half-reactions, and reactions in ionic form. | ||
| Adsorption of polymers on solid surfaces | unknown |
| Adsorption interaction at the interface and properties of boundary layers, boundary layers of polymers on solid surfaces, the influence of adsorption interaction on the molecular mobility of polymer chains in boundary layers, changes in the properties of boundary layers as a consequence of a decrease in molecular mobility, the influence of a solid surface on the supramolecular structures of polymers, adsorption connection polymers with adhesion, the influence of the interface on synthesis reactions and the structure of three-dimensional polymers. | ||
| Alchemy | unknown |
| Brief article on alchemy | ||
| Aldehydes and ketones | L.A. Tsvetkov |
| To make the place of aldehydes in the series of hydrocarbon oxidation products clear to students, when drawing up chemical equations one should not avoid using the names and formulas of the acids into which aldehydes are converted. The formulas of acids can be given dogmatically in advance... | ||
| Anthropogenic soil pollution | E.E.Matveev |
| Classification of soil contaminants, ways in which contaminants enter the soil, the importance of protecting soil cover. | ||
| Astatine | unknown |
| The history of the discovery of astatine, as well as its physical and chemical properties, is briefly outlined. | ||
| Atomic-molecular science in chemistry | G. P. Khomchenko |
| Atomic-molecular science was developed and first applied in chemistry by the great Russian scientist M.V. Lomonosov. The main provisions of his teaching are set out in the work “Elements of Mathematical Chemistry” (1741) and a number of others. The essence of Lomonosov’s teaching boils down to the following... | ||
| Acetylene. Acetylene production | L.A. Tsvetkov |
| Experiments in producing acetylene and studying its properties are demonstrated simultaneously. You should not prepare acetylene for the lesson in advance and store it in the gasometer due to the danger of explosion. The most accessible way to produce acetylene is the interaction of calcium carbide with water... | ||
| Benzene as a solvent | L.A. Tsvetkov |
| 1 ml of benzene is poured into one test tube, and the same amount of water into the other. A small piece of fat or a few drops of vegetable oil are placed in test tubes. The test tubes are shaken... | ||
| [ ] |
Toxicity[edit]
Eugenol is hepatotoxic, meaning it can cause liver damage. [25] [26] Overdose is possible, causing a wide range of symptoms, from blood in the patient's urine to seizures, diarrhea, nausea, loss of consciousness, dizziness, or rapid heartbeat. [27] According to a published report in 1993, a two-year-old boy nearly died after taking 5 to 10 ml. [28] Caution should be used when ingesting foods high in eugenol with MAO-inhibiting drugs. [23]
Bioactivity and toxicity
Eugenol and thymol have been found to have general analgesic properties. Like many other anesthetics, these 2-alkyl(hydroxy)phenols act as positive allosteric modulators of the GABA.A receptor. Although eugenol and thymol are too toxic and not effective enough for clinical use, these results led to the development of 2-substituted phenolic anesthetics, including propanidide (later withdrawn) and the widely used propofol.[22]
Eugenol is hepatotoxic, meaning it can cause liver damage.[23][24] Overdose is possible, causing a wide range of symptoms from blood in the patient's urine to seizures, diarrhea, nausea, unconsciousness, dizziness, or rapid heartbeat.[25] According to a published report in 1993, a two-year-old boy nearly died after taking 5 to 10 ml.[26]
Allergies [edit]
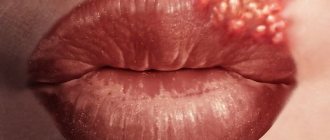
Eugenol is subject to restrictions on its use in perfumery [29] as some people may become sensitive to it, however, the extent to which eugenol may cause an allergic reaction in humans is controversial. [thirty]
Eugenol is a component of Balsam of Peru to which some people are allergic. [31] [32] When eugenol is used in dental preparations such as surgical pastes, dental tampons, and dental cement, it can cause contact stomatitis and allergic cheilitis. [31] Allergies can be detected using a patch test. [31]
Side effects, contraindications
Gevkamen ointment can provoke the development of allergies, which manifests itself in the form of tingling of the skin, a burning sensation or coldness at the site of application.
The use of this product is contraindicated in the presence of the following disorders:
- individual intolerance to individual components of the drug;
- when the epidermis is damaged in the inflamed area;
- when symptoms of dermatological pathologies appear;
- children under 12 years old.
During pregnancy or lactation, Gevkamen can be used only with the permission of the appropriate specialist and only if the expected beneficial effect from its use exceeds the potential risk to the child or fetus.
Natural phenomenon[edit]
Eugenol occurs naturally in several plants, including the following:
- Clove ( Syzygium aromaticum
) [33] [34] [35] - Wormwood [ link needed
] - Cinnamon [34] [36]
- Cinnamon tamala
[37] - Nutmeg ( Myristica Fragrans
) [38] - Ocimum basilicum
(sweet basil) [39] - Ocimum gratissimum
(African basil) [16] [40] - Ocimum tenuiflorum
(syn.
Ocimum sanctum
, tulsi or holy basil) - Japanese star anise [41]
- Melissa [42]
- Dill [ link needed
] - Pimenta dioica
(allspice) [
link
] - Vanilla [ link needed
] - Laurel Bay [ link needed
] - Celery [ link needed
] - Ginger [ link needed
]
- Wood advances
Eugenol.
Eugenol is a compound found in some plants such as basil, cinnamon, lemon and nutmeg, but it is primarily extracted from the clove plant. When it is extracted, it appears as a clear yellow liquid that smells strongly of cloves. It has medicinal value, but it is also used in products such as perfumes and flavored cigarettes. Although it is generally considered safe, eugenol may be harmful to people if it is used above recommended doses. In medicine, eugenol is used as an antiseptic and anesthetic. It is believed that it can reduce pain when applied to the skin or other injured parts of the body. Some men even apply the liquid to their genitals to prevent premature ejaculation. In dentistry, eugenol is often used in cavities used in restorative procedures and is rubbed into the gums to numb pain before dentures are inserted.
Eugenol is also widely used as an additive in a certain type of cigarette called clove cigarettes. It is also used to attract insects such as bees for research purposes. Some people value this compound because it also absorbs ultraviolet rays.
Despite its varied uses, eugenol can be dangerous, especially if taken more than the recommended dose. In some cases, the compound may cause liver damage. In other cases, it can cause cramps, nausea, rapid heartbeat, and dizziness. Some studies have shown that it can be toxic to the immune system, and many people believe that it can cause mutations in the body's DNA that lead to cancer. Eugenol is generally safe when taken with food. Researchers believe that the substance is not safe for children, nursing mothers, or pregnant women.
If a person is going to undergo surgery, it is usually recommended that the person stop using this mixture at least two weeks before surgery, as the compound slows down blood clotting. As a consequence, this can result in extreme and dangerous levels of bleeding on the surgical table. For the same reason, people who suffer from bleeding disorders; or have other bleeding disorders should not consume eugenol.
Although it can be prescribed by a dentist or doctor, eugenol can also be purchased at some pharmacies or online. If purchasing it online, it is important to ensure that it comes from a reputable source. Before using eugenol, any questions or concerns should be addressed by your consulting physician.