Principles of treatment of community-acquired pneumonia in adults
A summary of recommendations without analysis or commentary.
Diagnostics
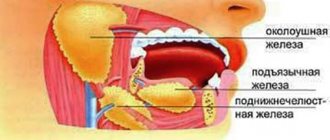
The diagnosis of community-acquired pneumonia is certain if the patient has radiologically confirmed focal infiltration of the lung tissue and at least two clinical signs from the following: a) acute febrile onset of the disease (T> 38.0°C); b) cough with sputum; c) physical signs (focus of crepitus and/or fine wheezing, hard/bronchial breathing, shortening of percussion sound); d) leukocytosis (>10·109/l) and/or band shift (>10%). In this case, it is necessary to take into account the possibility of known syndrome-like diseases/pathological conditions.
Gram stain and sputum culture
Routine sputum culture and Gram stain are not recommended for adult outpatients with community-acquired pneumonia.
In hospitalized patients, preliminary Gram stain and culture of airway secretions are recommended for adults with CAP that is considered severe (especially in intubated patients) or if CAP meets one of the following conditions:
- The patient is currently receiving empirical treatment for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- there has been a previous infection with MRSA or P. aeruginosa, especially a respiratory tract infection.
- the patient had been hospitalized within the previous 90 days and had received parenteral antibiotics for any reason.
Blood culture
Blood cultures are not recommended in adult outpatients with CAP.
Routine blood cultures are not recommended for hospitalized adult patients with CAP.
Pre-treatment blood cultures are recommended for hospitalized adult patients with CAP that is classified as severe or who meet one of the following conditions:
- Currently undergoing empirical treatment for MRSA or P aeruginosa
- Previous history of MRSA or P aeruginosa infection, especially of the respiratory tract
- Has been hospitalized within the previous 90 days and received parenteral antibiotics for any reason.
Test for Legionella antigen and pneumococcal antigen in urine
Routine urine testing for pneumococcal antigen is not recommended in adults with CAP unless the CAP is severe.
Routine testing of urine for Legionella antigen is not recommended in adults with CAP unless CAP is severe or there is indication of predisposing epidemiological factors (eg, a Legionella outbreak or recent travel).
Testing for Legionella should consist of urinary antigen assessment and collection of lower respiratory secretions for culture on selective media or nucleic acid amplification (PCR diagnostics).
Flu testing
If influenza virus is circulating in the community, testing for influenza in adult patients with CAP is recommended.
Determination of procalcitonin
Empirical antibiotic therapy is recommended for adult patients with a clinical picture of CAP and a radiologically confirmed diagnosis of CAP, regardless of the patient's initial serum procalcitonin level.
Treatment
The decision to hospitalize
The decision to hospitalize adults with CAP should be based primarily on an assessment of the severity of the disease (preferably using the Community-Acquired Pneumonia Severity Index (PSI) for Adults scale).
Direct admission to the intensive care unit is recommended for patients with CAP who have hypotension requiring vasopressor support or respiratory failure requiring mechanical ventilation.
Outpatient antibiotic treatment regimens
Antibiotics recommended for adult patients with CAP who are otherwise healthy:
- Amoxicillin 1 g three times daily OR
- Doxycycline 100 mg twice daily OR
- In areas with <25% pneumococcal macrolide resistance: macrolide (azithromycin 500 mg on day 1 and then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1000 mg daily)
For outpatient adults with CAP who have underlying medical conditions, the following antibiotic regimens are recommended:
- Combination therapy: Amoxicillin/clavulanate 500 mg/125 mg three times daily OR amoxicillin/clavulanate 875 mg/125 mg twice daily OR 2000 mg/125 mg twice daily OR cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily) PLUS
- Macrolide (azithromycin 500 mg on day 1, then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1000 mg once daily]) or doxycycline 100 mg twice daily OR
Inpatient antibiotic treatment regimens
The following empiric treatment regimens are recommended for adult patients with non-severe CAP who do not have MRSA or P. aeruginosa risk factors:
- Combination therapy with a beta-lactam (ampicillin plus sulbactam 1.5–3 g every 6 hours, cefotaxime 1–2 g every 8 hours, ceftriaxone 1–2 g daily or ceftaroline 600 mg every 12 hours) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily) OR
- Monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg per day, moxifloxacin 400 mg per day)
The following regimens are recommended for adult patients with severe CAP without MRSA or P. aeruginosa risk factors:
- Beta-lactam plus macrolide OR
- Beta-lactam plus respiratory fluoroquinolone
The use of antibacterial drugs active against anaerobic microorganisms is not recommended for suspected aspiration pneumonia, unless a lung abscess or empyema is suspected.
Antibacterial therapy with extended-spectrum drugs for MRSA or P. aeruginosa
Empiric antibiotics active against MRSA or P. aeruginosa are recommended for adult patients with CAP only in the presence of locally documented risk factors.
Empirical treatment options for MRSA include vancomycin (15 mg/kg every 12 hours) or linezolid (600 mg every 12 hours).
Empirical treatment options for P. aeruginosa include piperacillin-tazobactam (4.5 g every 6 hours), cefepime (2 g every 8 hours), ceftazidime (2 g every 8 hours), aztreonam (2 g every 8 hours) hours), meropenem (1 g each) 8 hours) or imipenem (500 mg every 6 hours).
Empiric therapy, based on the possibility of MRSA or P. aeruginosa, continues until laboratory culture data are available.
Corticosteroid therapy
Routine administration of corticosteroids is not recommended in adult patients with CAP or severe pneumonia associated with influenza. Their use is approved in patients with refractory septic shock.
Anti-influenza therapy
Anti-influenza treatment (eg, oseltamivir) should be given to all adults with CAP who test positive for influenza.
Antibacterial therapy in patients with influenza
Standard antibacterial treatment should be initially given to adults with clinical and radiological signs of CAP who test positive for influenza.
Duration of treatment
The duration of antibiotic therapy should be based on clinical data in the form of stabilization of the patient's condition and continue for at least 5 days after clinical improvement is achieved.
Criteria for the sufficiency of antibacterial therapy for pneumonia:
- Temperature < 37.5°C
- No intoxication
- No respiratory failure (respiratory rate less than 20 per minute)
- No purulent sputum
- The number of leukocytes in the blood < 10 x 109/L, neutrophils < 80%, juvenile forms < 6%
- No negative dynamics on the radiograph.
X-ray dynamics are observed more slowly compared to the clinical picture, so control chest X-ray cannot serve as a criterion for determining the duration of antibacterial therapy.
Follow-up chest imaging
Routine follow-up testing is not recommended for adult patients with CAP whose symptoms have resolved within 5 to 7 days.
Indications for hospitalization
In accordance with modern approaches to the management of adult patients with community-acquired pneumonia, a significant number of them can be successfully treated at home. In this regard, the following indications for hospitalization are of particular importance:
- Age over 60-65 years.
- The presence of concomitant diseases (chronic bronchitis/chronic obstructive pulmonary disease, bronchiectasis, malignant neoplasms, diabetes mellitus, chronic renal failure, congestive heart failure, chronic alcoholism, drug addiction, nutritional decline, cerebrovascular diseases).
- Hospitalizations (for any reason) that occurred within the last 12 months.
- Physical examination findings: respiratory rate ≥ 30/min; diastolic blood pressure ≤ 60 mm Hg. Art. ; systolic blood pressure <90 mm Hg. Art. ; heart rate ≥ 125/min; body temperature < 35.0°C or ≥ 40.0°C; disturbances of consciousness.
- Laboratory and radiological data: peripheral blood leukocyte count <4.0·109/l or >30.0·109/l; SaO2 < 92% (according to pulse oximetry), PaO2 < 60 mm Hg. Art. and/or PaCO2 > 50 mm Hg. Art. when breathing room air; serum creatinine > 176.7 µmol/l or urea nitrogen > 7.0 mmol/l (urea nitrogen = urea, mmol/l / 2.14); pneumonic infiltration localized in more than one lobe; presence of decay cavity(s); pleural effusion; rapid progression of focal infiltrative changes in the lungs (increase in the size of infiltration > 50% over the next 2 days); hematocrit <30% or hemoglobin <90 g/l; extrapulmonary foci of infection (meningitis, septic arthritis, etc.); sepsis or multiple organ failure, manifested by metabolic acidosis (pH < 7.35), coagulopathy.
- Inability to provide adequate care and follow all medical prescriptions at home.
- Preferences of the patient and/or family members.
Antibiotics of the 21st century
In the first part of the article, we talked about the main myths associated with antibiotics and described in detail the penicillin group, and now let’s move on to the next generation of antibiotics.
CEPHALOSPORINS
Microbes often act, if not wisely, then quite logically.
If they are threatened by penicillin antibiotics, the microbes begin to destroy penicillins (if only we could do this - something is bothering us, but we do it! - and wipe them off the face of the earth).
The weak link of penicillins is the so-called beta-lactam ring (you will often see this term in the description of drugs, so it is better to remember it). It is this beta-lactam ring that microbes have learned to break. And the tool for breaking is special enzymes, beta-lactamases.
So, in short, antibiotics of the cephalosporin group are the same penicillins, which work in exactly the same way, but they are not afraid of beta-lactamases. This means that they can deal with microbes that the same ampicillin or even amoxicillin with clavulanic acid cannot cope with.
The “arms race” between microbes and pharmacists over time gave rise to the second, third, and then fourth generations of cephalosporin antibiotics (when pronouncing some of the names of these drugs, even doctors are scared, immediately imagining what flora these antibiotics are intended against).
WHEN DOCTORS PRESCRIBE CEPHALOSPORINS
If you are allergic to regular penicillins.
Of course, the chemical structure of penicillins and cephalosporins is similar, but the chance that the patient will not have an allergic reaction to cephalosporins is still very high; If penicillin antibiotics do not treat the infection. This often happens if a patient becomes infected with some kind of staphylococcus or streptococcus already in the hospital: the microbes there are already poisoned by anything, and therefore are especially resistant to tablets from a regular pharmacy.
Microbes, unfortunately, learn quickly and evolve quickly. This is understandable: they only need 20 minutes to change generations.
And yet, doctors always recommend starting treatment with antibiotics from the penicillin group, so as not to breed antibiotic-resistant flora.
ANTIBIOTICS OF THE MACROLIDE GROUP
Penicillin revolutionized the treatment of infections. But very soon it turned out that he was capable of killing too. Here and there, patients began to die from anaphylactic shock caused by penicillin (remember - there are allergic reactions to penicillin?).
What could the scientists do? Just develop new antibiotics.
Penicillin began to be widely used in 1943 (in the USA and USSR, and almost simultaneously). And already in 1949, Alberto Aguilar in the Philippines discovered a new (after the green mold from which penicillin was isolated) special fungus that suppresses the growth of bacteria.
In the USA, a year later, another scientist, McGuire, isolated a new antibiotic from it - erythromycin (for some reason Wikipedia persistently writes that Eli Lilly isolated it, but this is not so - McGuire simply worked for him).
Erythromycin still works: it turned out that bacteria can adapt to it much less well than to penicillin.
WHAT CAN MACROLIDES DO?
Firstly, bacteria actually adapt to macrolides much more slowly than to penicillins.
But they also adapt. That is why erythromycin has now grown into a huge family of “younger brothers” - semisynthetic macrolides and azalides, which some pharmacists classify into another separate group - the fourth and last group of antibiotics used in pediatrics.
Secondly, macrolides do not kill bacteria - they deprive them of the ability to reproduce, as a result of which bacterial cells, without causing harm to humans, very quickly die by natural causes or become victims of the immune system.
Thirdly, macrolides can penetrate inside cells and overtake the bacteria that love to settle there - chlamydia and mycoplasma.
Chlamydia and mycoplasma do not have a cell wall, due to which they are invulnerable to penicillins (penicillins kill only bacteria that have a cell wall. If there is no cell wall, the task is to strangle Kolobok - it seems that you need it by the neck, but it simply isn’t there). But for macrolides, the absence of a cell wall is not an obstacle: chlamydia and mycoplasmas die upon contact with macrolides, although not so quickly.
WHEN DO DOCTORS PRESCRIBE MACROLIDES?
- the patient is allergic to penicillins;
Antibiotics stop working: pneumonia and sepsis set in
Head of the Department of Pulmonology at Sechenov University Sergei Avdeev told when patients need to be given medicine to avoid death
According to the results of a Levada Center survey in 2022, 34% of Russians believe that antibiotics kill viruses. This drug ignorance costs lives: bacteria become more and more resistant to drugs. As a result, about 700 thousand people die every year around the world from antimicrobial resistance (AMR, bacterial resistance to antibiotics - editor's note).
Sepsis and antimicrobial resistance: relevance for Russia
According to Roman Kozlov, corresponding member of the Russian Academy of Sciences, Doctor of Medical Sciences, professor, president of the Interregional Association for Clinical Microbiology and Antimicrobial Chemotherapy (IACMAC), the addiction of bacteria to antibiotics leads to both high mortality and a decrease in economic indicators.
“Global losses due to antibiotic resistance in 2022 amounted to about $400 million of GDP, and by 2050 they could grow to $8 trillion,” the expert noted. — Today, every year in the world, 700 thousand people die from resistance, and about 8 million 200 thousand from oncology. By 2050, the number of deaths from the consequences of AMR will increase to 10 million people per year.”
At the same time, fewer and fewer new antibiotics appear on the market every year. If in 1984 there were 18 of them, in 1998 - 12, then by 2012 their number had decreased to 4-5 per year. To change the situation with AMR, in 2022 the Russian government approved the “Strategy for preventing the spread of antimicrobial resistance in the Russian Federation for the period until 2030.”
“We are actively fighting the growth of AMR. And to do this, we inform the population that antibiotics only work for bacterial infections and are useless for viral ones. A professional standard “Medical Microbiology” was also developed and implemented, on the basis of which specialists working in this industry are now being trained,” said Kozlov. In addition, according to the expert, in Russia they are actively studying the mechanisms of the emergence of antimicrobial resistance; there is a special bank of bacterial strains.
Sepsis and pneumonia: prevention and treatment problems
According to Kozlov, as a result of the increase in antimicrobial resistance, the number of patients with, for example, pneumonia is increasing. In severe cases of this disease, blood poisoning occurs (sepsis), then saving the lives of patients is a matter of several days.
Sergey Avdeev, professor, head of the department of pulmonology, Sechenov University.
Professor of the Department of Hospital Therapy at Sechenov University, President of the Alliance of Clinical Chemotherapists and Microbiologists (Moscow) Sergei Yakovlev told how AMR can suddenly lead to the death of a person. One such case was the death of a 48-year-old woman who was taken to the hospital due to prolonged abdominal pain. At home, the patient took No-shpa and Analgin, but to no avail, so she called an ambulance. After hospitalization, the woman was diagnosed with appendicitis and peritonitis (inflammation of the abdominal cavity - editor's note), and she was operated on. Then a course of antibiotics was prescribed. But the patient was not getting better; the analysis showed the presence of Klebsiella in the body, a bacterium resistant to most antibiotics. The only antibiotic that could help was unavailable. Treatment with antibiotics according to different regimens did not help, and four days later the woman died of sepsis.
If in the 80s a number of bacteria were resistant to four groups of antibiotics, five still remained effective. Mortality was about 10–20%. From 2010 to the present time, almost all groups of antibiotics, except three, have become resistant to Klebsiella. As a result, in patients infected with this bacterium, the mortality rate is already 40–60%.
AMR causes problems for clinics
Among them is the rapid spread of multidrug-resistant bacteria in the department by patients with AMR, which significantly increases the risk of mortality of patients.
According to Sergei Yakovlev, to change the situation, it is necessary to introduce into the practice of medical organizations and programs for the rational use of antibiotics and curbing resistance. You should also quickly include all necessary antibiotics in the list of vital and essential drugs while they remain effective.
Sergei Yakovlev noted that another important measure is the revision of tariffs for payment for medical care in hospitals for the treatment of infections caused by multidrug-resistant pathogens within the framework of territorial compulsory medical insurance programs. “It is necessary to justify the constant availability and minimum balance in the hospital of all antibiotics for the treatment of multidrug-resistant infections (the “here and now” principle),” Yakovlev said.
Problems of access to effective antimicrobial therapy for sepsis
According to Sergei Zyryanov, Doctor of Medical Sciences, Professor, Deputy Head Physician for Therapy at City Clinical Hospital No. 24 of Moscow, in complex cases of pneumonia, hospital stay increases from 9 to 53 days.
In Russia, the average exchange rate cost of modern antibacterial therapy for antibiotic-resistant infections (monotherapy with an innovative drug or a combination of two to four traditional antibiotics) costs more than 300 thousand rubles. The expected exchange rate value of new antibacterial drugs, which are expected to be registered in the next two to three years (now available for use in other countries), is more than 900 thousand rubles.
“Today, tariffs for the provision of medical care for AMR do not cover the costs of health care facilities (health care facility is a medical and preventive institution), as a result, hospitals do not have the necessary medications at the right time and patients die,” says Zyryanov.
To avoid this you need to use an integrated approach. This is the introduction of an effective system for recording and recording infections caused by multidrug-resistant bacteria. Such an accounting will help to see the real number of deaths from sepsis in Russia. Then it is necessary to develop and include in the standards of medical care systems for additional financing of healthcare facilities' expenses for diagnostics and antimicrobial therapy of AMT.
In addition, a list of critical antimicrobial drugs that are required to be available in intensive care units of health care facilities should be developed and approved, and the payment model for innovative life-saving antimicrobial drugs (AMPs) for the treatment of infections caused by multidrug-resistant bacteria should be optimized.
Link to publication: cityreporter.ru